Preferential short cut or alternative route: the transaxillary access for transcatheter aortic valve implantation
Introduction
In recent years, transcatheter aortic valve implantation (TAVI) has been well established as a treatment option for patients with severe aortic stenosis who are at high or extreme surgical risk for conventional aortic valve replacement (1-3). For TAVI the most commonly used access route is the femoral artery. Yet, whenever the transfemoral approach is not feasible an alternative access has to be considered. The transapical, direct aortic, or transaxillary implantation routes currently serve as alternative access sites. Since its introduction, the latter approach was used only in selected cases. Lately, however, in experienced high volume centers it has been applied to a broader extent. In the present paper, we review the indication, access and implantation technique, procedural outcome, and future perspectives of the transaxillary approach for TAVI.
Indication
Peripheral vascular disease is a common finding in patients who are candidates for TAVI (2,4-6). For transvascular TAVI sheath sizes range from 14 to 20 F depending on valve size and type of device. Thus, for most devices a minimal vessel diameter of 5 to 6 mm is required. In our experience in at least 10 to 15% of TAVI patients a transfemoral approach seems to be not advisable due to significant peripheral artery disease or severe vessel tortuosity. According to BIBA (BIBA Med Tec, UK) in 2013, TAVI cases have been performed via transaxillary, direct aortic and transapical access in 1%, 4.7%, and 26.4%, respectively. Nevertheless, in some centers the transaxillary approach is used in up to 20% of the cases (7). Since alternative TAVI approaches have not been studied in a comparative fashion, the choice depends mainly on the experience and judgment of the heart team. Compared to other non-transfemoral approaches for TAVI, a strong argument for the transaxillary access is its lower invasiveness. Very much like for the transfemoral approach, it allows for a truly percutaneous delivery of the transcatheter heart valve (THV) (see section “Techniques of vessel access”). Also, it provides the opportunity to perform the intervention without general anesthesia, which might reduce the rate of anesthesia related complications such as failure of respiratory weaning or postoperative delirium and it may reduce length hospital stay and procedure time (8).
In the authors’ experience, to allow for safe deployment of the THV via the axillary and subclavian artery the following criteria have to be met:
- Diameter of the axillary and subclavian artery of ≥6 mm;
- Absence of heavy calcification, excessive kinking, or severe stenosis of the access vessel, which cannot be treated by balloon angioplasty;
- In patients with a patent left internal mammary artery (LIMA) coronary bypass graft, a minimal vessel diameter of 7-8 mm and no significant atherosclerotic disease proximal to or at the ostium of the LIMA in order to prevent myocardial ischemia.
By contrast, implanted pacemakers ipsilateral to the access site are not considered as a contraindication for the transaxillary approach.
To adequately evaluate the transaxillary access route, a multislice computed tomography scan should be performed prior to implantation in any case. Angiography or duplex sonography of the axillary and subclavian artery may provide additional useful information in borderline cases.
Techniques of vessel access
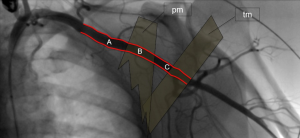
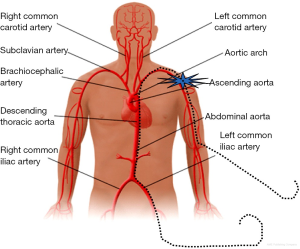
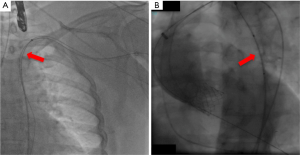
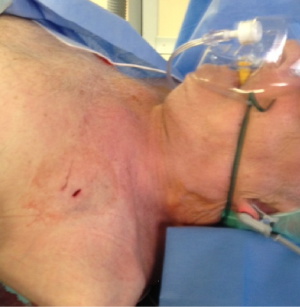
Figure 1 illustrates the anatomy of the axillary artery. The subclavian artery runs above the first rib and subsequently becomes the axillary artery, which then courses posterior to the pectoralis minor muscle and becomes the brachial artery at the inferior border of the teres minor muscle. The ideal access site of the axillary artery is in its first segment between the lateral border of the first rib and the medial border of the pectoralis minor muscle due to the absence of major side branches. The left axillary artery is preferred, because it allows for coaxial orientation of the valve prosthesis within the aortic annulus. An angle >30° between the annular plane and the horizontal axis (i.e., a horizontal orientation of the annulus) should be considered as a significant limitation for the right sided access. In general, the right axillary artery should only be chosen if the left side is not accessible. The axillary artery can be reached either by surgical cut-down or by direct percutaneous puncture. Most centers prefer a surgical cut-down of the artery. In this case, the puncture site is prepared with a double purse-string suture. Alternatively, arteriotomy and placement of a Goretex conduit is performed to provide access to the vessel. However, also a truly percutaneous approach without the need for vascular surgery is possible (Figures 2-4). For this purpose, a long safety-wire (e.g., the 4 meter Ply-wire™, OptiMed; Ettlingen, Germany) has been proposed to be placed into the axillary artery via the ipsilateral brachial artery. The wire is subsequently snared and externalized through the femoral artery (Figure 2) (9). The wire serves as a landmark for fluoroscopic guided puncture at a spot below the clavicle and lateral to the rib cage, to eliminate the risk for pneumothorax. Adequate closure of the axillary artery is the most critical issue when performing the percutaneous approach, because, due to the anatomical conditions, in most instances manual compression of the puncture site is not efficient. Consequently, untreated vessel leakage may rapidly lead to hemorrhagic shock. Vessel closure can safely be performed using a vascular preclosure device. For this purpose two ProGlide 6 F devices (Abbott Vascular, Abbott Park, IL, USA) have been found to be more effective than the ProStar XL 10 F device (Abbott Vascular). The device is inserted into the axillary artery just after vessel puncture and the sutures are left untied until the end of the procedure (pre-closure). To prevent complications in the wake of closure device failure, measures for an adequate endovascular bail-out maneuver should always be provided. Accordingly, placing an uninflated PTA balloon via the femoral artery over the externalized long wire in the descending aorta before insertion of the valve introducer sheath into the axillary artery is highly recommended (Figure 3) (9). The long wire can serve as a monorail endovascular access loop and enables vascular blockade with a balloon, stent graft treatment, or both, either from the femoral or the brachial site, or in a rendezvous fashion even in cases of severe vascular damage such as rupture or severe dissection. This technique is also recommended for the surgical cut-down of the axillary artery in case an unreachable injury at the proximal subclavian artery occurs. Regardless of the surgical or the percutaneous approach, after adequate preparation of the access site, the procedure is performed by following the recommendations similar to the transfemoral implantation technique, which has been described previously (1,10).
Outcome
Data regarding patient outcome after transaxillary TAVI is scarce. Prospective, randomized studies comparing the transaxillary access with other approaches for TAVI are completely lacking. The first report of successful transaxillary TAVI in 3 patients was published in 2009 by the group of Fraccaro and co-workers (11). The patients in this series were considered inoperable with a logistic EuroSCORE I ranging from 25.2% to 53.8%. In all patients TAVI was performed with the CoreValve Revalving System (Medtronic Inc.; Minneapolis, MN, USA). Procedural success was achieved in all three cases with no adverse events at 3-month follow-up. In 2010, Petronio and co-workers published multicenter registry data of 54 high-risk patients, in whom the CoreValve THV was implanted by transaxillary access by surgical cut-down (12). Compared to a transfemoral group enrolled in the same registry the calculated risk was significantly higher in the transaxillary group [mean logistic EuroSCORE I of 25.3% (interquartile range, 15.1-36.6%) vs. 19.4% (interquartile range, 12.5-19.8%); P=0.03]. Not surprisingly, the higher risk was mainly caused by a higher prevalence of coronary, cerebral, and peripheral artery disease. Procedural and clinical outcome of transaxillary TAVI was excellent with a success rate for device implantation of 100%, no reported deaths at 30 days, and freedom from major adverse valve-related events after 6 months in 88.5%. There was no significant difference in procedural success and mortality between the transaxillary and the transfemoral group. In a propensity-matched comparison of the transaxillary and the transfemoral approach this finding was confirmed for 2-year outcomes with even lower rates of acute kidney injury, minor vascular complications and bleeding events in the transaxillary group (7). In consideration of these results, the authors concluded that the transaxillary approach might also be indicated if the transfemoral access is difficult but still feasible. The surgical cut down, performed in the transaxillary group, may have contributed to the longer procedure time compared to the percutaneously treated transfemoral group, a finding, which was also observed in a German single center analysis (13). Reduction in procedural time and invasiveness might be achieved by a truly percutaneous transaxillary approach for TAVI. A single center study comprising 24 high risk patients (mean logistic EuroSCORE I 35.3%±22.8%) treated with the percutaneous technique revealed a device success in 95.8% (23/24) of patients and a 30 day mortality rate of 8.4% with no major adverse cardiac and cerebrovascular events and no major vascular complications according to the Valve Academic Research Consortium (VARC1) criteria (9). However, closure device failure was seen in 29.2% of patients, who subsequently received stent graft implantation. In all cases with failed vessel closure, the ProStar system had been used, whereas use of the ProGlide system had resulted in a 100% vessel closure success rate. Such a difference in performance of the two closure device systems seems to be specific for the transaxillary approach, since it has not been reported for the transfemoral approach, where use of either system is recommended (14). The superiority of the ProGlide system for the percutaneous transaxillary approach may be explained by the different vessel wall properties of the axillary artery (elastic type) compared to the femoral artery (muscular type) and the less traumatic nature of the ProGlide system compared to the ProStar system (9).
Future perspectives
Transaxillary access for TAVI is technically feasible and results in clinical outcomes comparable to those after transfemoral TAVI, even though patients, who are treated via the axillary artery, usually present with an increased clinical risk profile. However, we believe that the transfemoral approach will remain the standard implantation route for THVs, because, compared to the transaxillary approach, vascular access is easier to provide and progress is still being made in terms of reduction of device-size, lowering the minimum vessel diameter required for TAVI. Nevertheless, considering the good results regarding technical success and patient outcome, the transaxillary implantation route should always be taken into account whenever the heart team needs to decide on an alternative access site for TAVI in patients who are not suitable for transfemoral access. Still, if the low invasiveness provided by the transaxillary access translates into an improved clinical outcome compared to transapical or direct aortic approaches has to be verified in a comparative, prospective, randomized trial in the future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [PubMed]
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [PubMed]
- Moat NE, Ludman P, de Belder MA, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol 2011;58:2130-8. [PubMed]
- Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation 2011;123:299-308. [PubMed]
- Kurra V, Schoenhagen P, Roselli EE, et al. Prevalence of significant peripheral artery disease in patients evaluated for percutaneous aortic valve insertion: Preprocedural assessment with multidetector computed tomography. J Thorac Cardiovasc Surg 2009;137:1258-64. [PubMed]
- Petronio AS, De Carlo M, Bedogni F, et al. 2-year results of CoreValve implantation through the subclavian access: a propensity-matched comparison with the femoral access. J Am Coll Cardiol 2012;60:502-7. [PubMed]
- Motloch LJ, Rottlaender D, Reda S, et al. Local versus general anesthesia for transfemoral aortic valve implantation. Clin Res Cardiol 2012;101:45-53. [PubMed]
- Schäfer U, Ho Y, Frerker C, et al. Direct percutaneous access technique for transaxillary transcatheter aortic valve implantation: "the Hamburg Sankt Georg approach JACC Cardiovasc Interv 2012;5:477-86. [PubMed]
- Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014;63:1972-81. [PubMed]
- Fraccaro C, Napodano M, Tarantini G, et al. Expanding the eligibility for transcatheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system. JACC Cardiovasc Interv 2009;2:828-33. [PubMed]
- Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010;3:359-66. [PubMed]
- Muensterer A, Mazzitelli D, Ruge H, et al. Safety and efficacy of the subclavian access route for TAVI in cases of missing transfemoral access. Clin Res Cardiol 2013;102:627-36. [PubMed]
- Toggweiler S, Leipsic J, Binder RK, et al. Management of vascular access in transcatheter aortic valve replacement: part 1: basic anatomy, imaging, sheaths, wires, and access routes. JACC Cardiovasc Interv 2013;6:643-53. [PubMed]


