
Acceptability and feasibility of S-1 plus cisplatin adjuvant chemotherapy for completely resected non-small cell lung cancer: an open-label, single arm, multicenter, phase 2 trial
Introduction
Lung cancer is a major cause of cancer death worldwide (1). Globally, there were 20 million new cases of tracheal, bronchial, and lung cancer and 1.7 million deaths from these conditions in 2016, representing a 28% increase in incidence between 2006 and 2016 (2); thus, new developments in lung cancer treatment are essential.
According to a database of patients who underwent surgery in Japan (3), the 5-year survival rates of patients with stage IIA, IIB, and IIIA disease (reclassified with respect to clinical stage from the TNM classification seventh edition to the eighth edition) were 60.2%, 58.1%, and 50.6%, respectively. Distant metastasis was the most common form of recurrence in patients who had undergone complete resection, indicating that control of micrometastases at the time of resection improves prognosis of these patients. Therefore, adjuvant chemotherapy is the accepted standard of care, largely based on the results of several randomized trials and meta-analyses (4-6). Adjuvant vinorelbine plus cisplatin is widely accepted as a standard regimen for completely resected non-small cell lung cancer (NSCLC). Although a subgroup analysis (7) of the LACE study (6) reported an 8.9% 5-year survival improvement compared to surgery alone, the adjuvant therapy completion rate was 43% due to patient refusal and adverse events. Adverse events sometimes require reduced chemotherapy doses or even discontinuation. Among patients with stage I-III NSCLC, it has been reported that 42% of patients were expected to receive chemotherapy at a relative dose intensity (RDI) <85% of the standard dose due to dose-limiting toxicities, but 63% of patients actually received an RDI <85% (8). The inability to provide patients with a sufficient amount of drug may limit their survival outcomes.
S-1 is a tegafur-uracil-based, second-generation anticancer agent that contains tegafur, gimeracil (an inhibitor of dihydropyrimidine dehydrogenase, which decomposes fluorouracil), and oteracil (which inhibits the phosphorylation of fluorouracil in the gastrointestinal tract, thus decreasing associated gastrointestinal toxicity) in a 1:0.4:1 ratio (9). Administration of S-1 monotherapy following complete resection of stage IB-IIIA NSCLC has been shown to be safe, with adequate compliance (10). A randomized phase 3 study in Japanese patients with advanced NSCLC (11) demonstrated that survival associated with S-1 plus cisplatin was not inferior to that of docetaxel plus cisplatin, and both adverse events and quality of life were superior among patients who received S-1 plus cisplatin. To sufficiently enhance the efficacy of chemotherapy, it is necessary to administer a sufficient amount of anticancer drugs. Despite the introduction of new agents and regimens, 5-year survival had improved <10%, and new treatment strategies and drugs are required to further improve the prognosis of patients with resectable NSCLC.
The objective of this study was to investigate the acceptability and feasibility of using 4 courses (5-week cycles) of combination therapy with S-1 and cisplatin as postoperative adjuvant chemotherapy following complete resection of pathological stage II-IIIA NSCLC.
We present the following article in accordance with the TREND reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-3293).
Methods
Eligibility criteria
Patients with confirmed pathological stage II–IIIA NSCLC who had not received preoperative chemotherapy or radiotherapy and had undergone surgical treatment were enrolled. The eligibility criteria were: (I) pathologically confirmed NSCLC, (II) pathological stage II–IIIA (UICC-7 TNM lung cancer staging, 2009) disease, (III) pathological complete resection, (IV) performance of ND2a or more extensive lymph node dissection, (V) no prior treatment other than surgery, (VI) Eastern Cooperative Oncology Group performance status (PS) 0 or 1, (VII) age ≥20 years and <75 years, (VIII) no functional impairment of major organs, and laboratory test results showing WBC ≥3,000/mm3, leukocytes ≥1,500/mm3, platelets ≥105/mm3, hemoglobin ≥9.0 g/dL, AST ≤100 IU/L, ALT ≤100 IU/L, total bilirubin ≤1.5 mg/dL, and CCr ≥60 mL/min, (IX) PaO2 ≥65 mmHg or SpO2 ≥92%, and (X) able to undergo chemotherapy within 8 weeks after surgery.
The exclusion criteria were: (I) serious drug allergy, (II) currently undergoing treatment with flucytosine, (III) pulmonary fibrosis or interstitial pneumonia, (IV) infection requiring antibiotic treatment or poorly controlled complications, (V) serious diarrhea, (VI) malignancy within the previous 5 years, (VII) previous complete pneumonectomy, or (VIII) judged by the attending physician to be unsuitable for the study.
Study design and treatment
This study was a multicenter phase 2 clinical trial, for which registration started in February 2013 in five centers in Japan. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Toho University Omori Medical Center (approved number: M17050) and was registered on the UMIN clinical study registration site (protocol ID: UMIN000016191) (Retrospectively registered). Written informed consent was obtained from all patients. Patients were treated with S-1 twice daily for 21 days at an initial dose determined by body surface area (BSA) [BSA <1.25 m2: 40 mg/dose (80 mg/day); 1.25 ≤ BSA <1.5 m2: 50 mg/dose (100 mg/day); BSA ≥1.5 m2: 60 mg/dose (120 mg/day)] followed by a 14-day drug holiday, during which they were also given cisplatin 60 mg/m2 on day 8. This 35-day period constituted 1 cycle, and treatment was repeated for 4 cycles. Pre- and post-treatment hydration and antiemetics were administered in accordance with the standard methods employed at each site. The study was continued, except in the following situations: when 21 days had elapsed after the scheduled start date of treatment, when the dose needed to be reduced due to a further adverse event after the two-step dose reduction of S1, when recurrence was observed, when the patient requested withdrawal of treatment, or when the attending physician judged that the trial should not be continued for any other reason.
Outcome measures and statistical methods
The primary endpoint was the rate of completion of 4 treatment courses, and the secondary endpoints were safety, status of drug administration, disease-free survival (DFS), and overall survival (OS). OS was defined as the time from the date of surgery to death from any cause or final follow-up. DFS was defined as the time from the date of surgery to the date recurrence was determined or death from any cause, whichever came first, or the time to final follow-up. Safety was evaluated by calculating the frequency of the highest-grade adverse events, assessed by CTCAE v4.0-JCOG, in the analysis set comprising all patients who underwent treatment. The status of drug administration was evaluated by calculating the dose administered to patients and accounting for factors including drug holidays, dose reductions, and skipped doses, in the analysis set including all patients who underwent treatment. The total anticipated doses and actual dosage rates in this study were calculated as follows:
- S-1: total anticipated dose = initial dose (mg/day) × total number of scheduled treatment days (days), actual dosage rate (%) = total dose administered during the treatment period (mg)/total anticipated dose (mg) ×100%
- Cisplatin: total anticipated dose = 240 mg/m2, actual dosage rate (%) = total dose administered during the treatment period (mg)/240 (mg/m2) ×100%
The Kaplan-Meier method was used to describe DFS and OS. All statistical analysis was performed using JMP® Pro 14 (SAS Institute., Cary, NC, USA).
Results
Patient characteristics
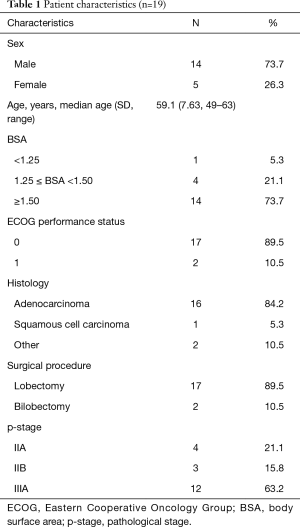
A total of 22 patients at 5 sites were enrolled between February 2013 and September 2017. Three were excluded as ineligible, and the patient characteristics of the remaining nineteen are shown in Table 1. They included 14 men and 5 women, with a mean age of 59.1 years (range, 49–63 years). All patients underwent lobectomy, including two cases of bilobectomy. A total of 7 patients (36.8%) had stage II disease and 12 patients (63.2%) had stage IIIA disease, and the pathological diagnosis was adenocarcinoma in 16 cases. The BSA was <1.25 m2 in 1 case, 1.25 ≤ BSA <1.5 m2 in 4 cases, and BSA ≥1.5 m2 in 14 cases.
Full table
Treatment delivery and safety
Patients in this study underwent a total of 60 cycles. The completion rates and implementation status are shown in Table 2. Fourteen (68.4%) patients completed 4 cycles. Three patients discontinued the study due to adverse events, which included grade 3 gastrointestinal symptoms (nausea) in all cases during the first cycle. Those patients refused to continue in the trial without accepting a dose reduction in the next cycle. Recurrence was detected after the completion of 2 cycles in 1 patient (the sites of recurrence were the lung and the brain) and after the completion of 3 cycles in 1 patient (the patient developed carcinomatous pleurisy). Furthermore, 1 patient discontinued treatment during the second cycle due to another disease (ruptured aortic aneurysm). The administration status of S-1 plus cisplatin is described in Table 3. Among patients who received more than 2 cycles, planned dose reduction was required in only 2 cases, with the doses of both S-1 and cisplatin reduced in 1 patient and only the dose of S-1 reduced in the other patient; both of these patients completed 4 cycles. The median RDI was 79%±32% for S-1 and 80%±32% for cisplatin. Overall, 68.4% of patients achieved an actual RDI ≥85%.

Full table

Full table
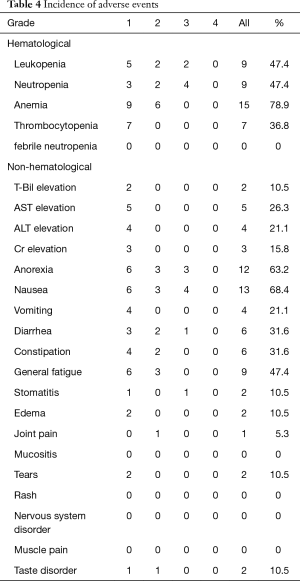
Table 4 lists the adverse events that occurred in all patients. Evaluated by worst grade, the most common adverse event was anemia (78.9%), followed by nausea (68.4%) and anorexia (63.2%). Grade 3/4 adverse events included neutropenia (21.1%), nausea (21.1%), anorexia (15.8%), and leukopenia (10.5%); no grade 4 adverse event of any kind occurred, and there were no deaths due to adverse events.
Full table
Efficacy
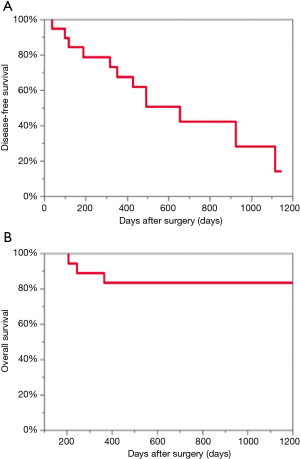
The median follow-up for the 19 patients was 926 days (95% CI: 646–1,034 days). Median DFS was 656 days (95% CI: 318–1,116 days), and median OS could not be calculated due to the short observation time. Two-year DFS was 42.1%, and two-year OS was 83.3% (Figure 1). During the follow-up period, 12 patients developed recurrence and 4 patients died. Of the 12 patients who developed recurrence, 9 patients developed single organ metastasis (4 patients developed lung metastasis, 1 patient developed carcinomatous pleurisy, 2 patients developed hilar mediastinal lymph node metastases, 1 patient developed brain metastasis, and 1 patient developed bone metastasis) and 3 patients developed multiple metastases (1 patient developed lung and liver metastases, 1 patient developed lung and brain metastases, and 1 patient developed adrenal, mediastinal hilum, and abdominal lymph node metastases).
Discussion
This study demonstrated the feasibility of combination therapy with S-1 plus cisplatin as postoperative adjuvant chemotherapy following complete resection of pathological stage II–IIIA NSCLC. The 4-cycle completion rate, the primary endpoint of this clinical trial, was 68.4%, and no grade 4 adverse events or deaths occurred throughout the 4 cycles. The most common grade 3/4 adverse events were neutropenia and gastrointestinal adverse events including nausea, vomiting, anorexia, and diarrhea. In addition, febrile neutropenia (FN) was not observed in any patient.
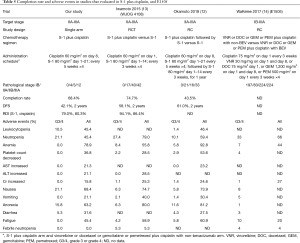
Two Japanese clinical trials of postoperative adjuvant chemotherapy with S-1 plus cisplatin have been reported (12,13) (Table 5 shows the results of the present clinical trial and these two Japanese clinical trials). A phase 2 randomized trial comparing S-1 vs. S-1 plus cisplatin (WJOG4108 trial) (13) reported a 4-cycle completion rate of S-1 plus cisplatin arm of 74.7% and a 2-years DFS rate of 61%. The incidence of grade 4 neutropenia was 5.3% and that of grade 3 FN was 5.3%, but there were no cases of grade 4 FN. The dosing schedule of S-1 plus cisplatin was S-1 80 mg/m2 on 14 consecutive days and cisplatin 60 mg/m2 on day 1, and the study population included 58.2% patients with stage II disease and 41.8% with stage III disease. The other phase 2 randomized trial, which evaluated S-1 vs. S-1 plus cisplatin followed by S-1 (Okamoto et al.) (12), reported a completion rate of S-1 plus cisplatin followed by S-1 of 45.7% and a 2-years DFS rate of 58.4%. The incidence of grade 3/4 neutropenia was 10.1%, and no treatment-related deaths occurred. The dosing schedule of S-1 plus cisplatin followed by S-1 was S-1 80 mg/m2 on 21 consecutive days and cisplatin 60 mg/m2 on day 8 every 5 weeks, followed by S-1 80 mg/m2 on 14 consecutive days for 1 year, and registered patients included 62.3% with stage II disease and 37.7% with stage III disease. The 2-years DFS rate in the study was 42.1%. Although our study included a small number of patients, the DFS rates in our study were lower than those reported in previous studies. One reason for this was the number of patients with advanced cancer registered in each study. More than half of the patients in our study had pathological stage IIIA disease. Another reason was the differences in the S-1 plus cisplatin administration schedules between our study and studies. The key drug cisplatin was administered at an interval of 3 weeks in the WJOG 4108 trial; however, it was administered at an interval of 5 weeks in our study and the study by Okamoto et al. In the study by Okamoto et al., the 2-year DFS was 61%, but many patients developed early recurrence within 1 year after surgery. Administration of cisplatin at short intervals may help prevent early recurrence. Although the completion rate was not high in the study by Okamoto et al., subsequent maintenance therapy suppressed recurrence. However, despite the differences in the S-1 plus cisplatin administration schedules between these trials, no treatment-related deaths occurred.
Full table
Neutropenia is the major dose-limiting toxicity of many chemotherapy regimens. FN is defined as neutropenia with fever, usually indicative of infection, and can be fatal. The mortality rate associated with FN has been estimated to be 9.5% (15). In a nationwide prospective cohort study in the United States (8), FN occurred in 6.4% of patients in the first cycle of myelosuppressive chemotherapy, and cumulative events occurred over 4 cycles in 11% of patients. The JBR.10 study (inclusion period, 1994–2001) (4) and the ANITA study (inclusion period, 1994–2000) (5), which were analyzed in the LACE trial and led to the wide acceptance of the adjuvant vinorelbine plus cisplatin regimen, reported 73% and 85% rates of grade 3 and 4 neutropenia, respectively, and 7% and 9% rates of grade 3 and 4 FN, respectively. Recently, the results of the E1505 trial (inclusion period, 2007–2013) (14) were published. This study was a phase 3 randomized trial that compared the combination of vinorelbine, docetaxel, gemcitabine, and pemetrexed plus cisplatin with and without bevacizumab (BEV) as postoperative adjuvant chemotherapy after resection of pathological stage IB-III NSCLC. Not only was the addition of BEV ineffective in improving prognosis, but grade 3–5 adverse events occurred more frequently in the BEV arm. Rates of grade 3–5 neutropenia were 33% and 37% in the non-BEV arm and the BEV arm, respectively, and FN rates were 4% and 6%, respectively. Thus, this study demonstrated that BEV should not be included in postoperative adjuvant chemotherapy regimens. Nevertheless, we gained important clinical value from that trial, because it was a cisplatin-based study with patient enrollment occurring from 2007–2013, and the precautionary measures and management methods for adverse events used were similar to those of the study. Although the use of granulocyte-colony stimulating factor (G-CSF) was not reported, the incidence of adverse events is useful for comparison with those associated with S-1 plus cisplatin regimens (Table 5).
To sufficiently enhance the efficacy of chemotherapy, it is necessary to administer sufficient amounts of anticancer drugs. However, adverse events sometimes necessitate a reduction in the dosage of the drug and may require drug discontinuation. As mentioned in the Introduction, among patients with stage I–III NSCLC, it has been reported that 42% of patients are expected to receive chemotherapy at an RDI <85% of the standard dose due to dose-limiting toxicities, but 63% of patients actually received an RDI <85% (8). In the study, 53% of patients were able to receive the planned dose, and 32% of patients received an overall actual RDI <85%. Thus, S-1 plus cisplatin appears to be a highly safe adjuvant chemotherapy regimen when administered using current standard precautionary measures for adverse events.
Clinical trials of S-1 plus cisplatin have tended to have high rates of gastrointestinal adverse events, and S-1 alone is known to be associated with a high incidence of gastrointestinal adverse drug reactions. In a randomized clinical trial that compared the feasibility of 4 weeks of S-1 monotherapy followed by a 2-week drug holiday with 2 weeks of S-1 monotherapy followed by a 1-week drug holiday as adjuvant chemotherapy (16), rates of nausea (all grades) were 39% vs. 38%, respectively, and those of anorexia were 37% vs. 35%, respectively, with no difference in incidence of gastrointestinal toxicity between the two treatment arms. In the E1505 study (14), however, the incidence of gastrointestinal toxicity was <10%, indicating that this form of toxicity is not necessarily characteristic of S-1. In the study, 3 of 19 patients discontinued treatment during the first cycle due to gastrointestinal symptoms. Although it is unclear whether their reasons for dropping out were the dose of S-1 or the administration method, when S-1 is used in combination with cisplatin, the measures to improve gastrointestinal toxicity are required beginning soon after the start of administration.
Finally, based on the results of two previous studies (12,13) and our study, S-1 plus cisplatin is a safe regimen with a low risk of death due to treatment. However, further development of postoperative chemotherapy regimens is necessary to achieve lower adverse events and a better prognosis.
Conclusions
This study demonstrated S-1 plus cisplatin is associated with a low risk of life-threatening adverse events, such as FN. S-1 plus cisplatin may be a highly safe adjuvant chemotherapy regimen when given in conjunction with current precautionary measures for adverse events.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-3293
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-3293
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3293). AI serves as an unpaid editorial board member of Journal of Thoracic Disease from May 2019 to Apr 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Toho University Omori Medical Center (approved number: M17050) and was registered on the UMIN clinical study registration site (protocol ID: UMIN000016191) (Retrospectively registered). Written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Okami J, Shintani Y, Okumura M, et al. Demographics, Safety and Quality, and Prognostic Information in Both the Seventh and Eighth Editions of the TNM Classification in 18,973 Surgical Cases of the Japanese Joint Committee of Lung Cancer Registry Database in 2010. J Thorac Oncol 2019;14:212-22.
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [Crossref] [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Douillard JY, Tribodet H, Aubert D, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol 2010;5:220-8. [Crossref] [PubMed]
- Culakova E, Thota R, Poniewierski MS, et al. Patterns of chemotherapy-associated toxicity and supportive care in US oncology practice: a nationwide prospective cohort study. Cancer Med 2014;3:434-44. [Crossref] [PubMed]
- Shirasaka T, Shimamato Y, Ohshimo H, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 1996;7:548-57. [Crossref] [PubMed]
- Okumura S, Sasaki T, Satoh K, et al. Feasibility of adjuvant chemotherapy with S-1 consisting of a 4-week administration and a two-week rest period in patients with completely resected non-small cell lung cancer. Mol Clin Oncol 2013;1:124-30. [Crossref] [PubMed]
- Kubota K, Sakai H, Katakami N, et al. A randomized phase III trial of oral S-1 plus cisplatin versus docetaxel plus cisplatin in Japanese patients with advanced non-small-cell lung cancer: TCOG0701 CATS trial. Ann Oncol 2015;26:1401-8. [Crossref] [PubMed]
- Okamoto T, Yano T, Shimokawa M, et al. A phase II randomized trial of adjuvant chemotherapy with S-1 versus S-1 plus cisplatin for completely resected pathological stage II/IIIA non-small cell lung cancer. Lung Cancer 2018;124:255-9. [Crossref] [PubMed]
- Iwamoto Y, Mitsudomi T, Sakai K, et al. Randomized Phase II Study of Adjuvant Chemotherapy with Long-term S-1 versus Cisplatin+S-1 in Completely Resected Stage II-IIIA Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:5245-52. [Crossref] [PubMed]
- Wakelee HA, Dahlberg SE, Keller SM, et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 2017;18:1610-23. [Crossref] [PubMed]
- Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006;106:2258-66. [Crossref] [PubMed]
- Hata Y, Kiribayashi T, Kishi K, et al. Adherence and feasibility of 2 treatment schedules of S-1 as adjuvant chemotherapy for patients with completely resected advanced lung cancer: a multicenter randomized controlled trial. BMC Cancer 2017;17:581. [Crossref] [PubMed]


