Muscle dysfunction in chronic obstructive pulmonary disease: update on causes and biological findings
For the last one and a half million years, our species has been moving and breathing on earth (1). In addition, we have been able to modify our environment, develop tools and skills and, thereby, create what today we call culture. All these functions essential for both individual survival and the biological success of our species are linked to the contractile properties of striated muscles. Their name derives from their structure, since their basic functional unit, the sarcomere, gives them a typical striped appearance. When, as a result of a disease or even a physiological process (such as ageing) our muscles do not work properly, we become weak and frail and may even die.
Different muscles, different tasks
However, the tasks of the muscles are not homogeneous. Some of them, such as those located in the pelvic girdle and lower limbs are specialized in enabling us to move around. Whereas the muscles located in the upper limbs and scapular girdle are essential for the manipulation of all kinds of objects and also for self-care activities (2,3). Both the upper and lower limb muscles are also known as “peripheral” muscles. Like those located in the trunk they are also referred to as “skeletal muscles” since they move the bones by means of their joints, thus producing movement and/or maintaining the skeleton structure. Other striated but very specialized muscles, whose role is to provide the subject with alveolar ventilation through breathing movements, are known as respiratory muscles. The latter can be subdivided into either inspiratory or expiratory muscles, depending on the part of the ventilatory cycle (inspiration or expiration, respectively) where their activity is more predominant. Inspiratory muscle contraction generates changes in thorax shape and volume, which combined with natural retraction of the lungs, increases the negativity of intrathoracic pressure. The gradient between intrathoracic and atmospheric pressures determines the airflow to the lungs. The main inspiratory muscles are the diaphragm (which can be subdivided into costal and crural portions), external intercostals and parasternals (4-10). However, many other muscles such as scalenes, sternocleidomastoid, latissiums dorsi, serratus and pectoralis can also participate progressively in the breathing effort if ventilatory loads or demands increase and/or the main inspiratory muscles fail to perform their job properly (11-14). Under normal conditions, expiration is a much simpler process since the mere relaxation of inspiratory muscles results in the reduction of the negativity of pleural pressure and a slightly positive alveolar pressure. This gives rise to the exit of the air from the respiratory system. However, some muscles can facilitate exhalation if necessary, and are therefore known as expiratory muscles (9,15-18). The main expiratory muscles are those that make up the abdominal wall (mostly major and minor obliques and transverse abdominis) as well as the internal intercostal group, with the exception of parasternals (which as mentioned before act predominantly during inspiration). Finally, another very specialized and critical muscle is the myocardium. Its contraction is responsible for blood flow through the entire body, including not only the perfusion of peripheral tissues but also pulmonary circulation, which is essential for gas exchange. However, its function has become so specialized that the muscle phenotype has diverged from that of classical striated muscles, to the point where it is classified as a different category: the cardiac muscle.
Muscle function and dysfunction
Striated muscles have two main functional properties: strength, or the ability to develop a maximal effort, and endurance, or the ability to maintain a submaximal effort through time. Therefore, it is worth noting that the time-dependence (shorter for strength and longer for endurance) and the magnitude of the effort (maximal in the case of strength and submaximal in that of endurance) are the two main differences between these concepts. Muscle dysfunction can be defined as the situation where skeletal muscles show reduced strength and/or reduced endurance, being unable to perform their physiological tasks adequately. Muscle dysfunction can be expressed both as fatigue or weakness. Fatigue is a state in which the muscle is temporarily unable to perform its current tasks (Figure 1). This condition is reversible with rest, thus differing from the concept of weakness, which is a much more permanent impairment in muscle contractile properties. Although fatigue and weakness appear to be very different conditions, they are related in the sense that a weak muscle becomes more easily fatigued. There are different conditions, and not only diseases, which can result in muscle dysfunction. Some involve striated muscle structure directly while others primarily affect the structures of the nervous, vascular and osteoarticular systems (18,19).
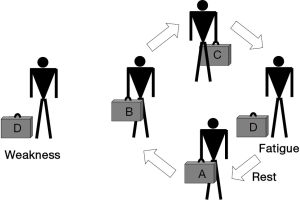
When lower limb muscles become ineffective in doing their tasks, the mobility of the individual is reduced. This in turn generates the perception of being disabled, and has an extraordinary impact on one’s quality of life. A similar effect results from the loss of function in the upper limb muscles, since subjects would not only be unable to maintain their professional lives but, at advanced stages, they would also require assistance even for the simplest of everyday tasks. If the respiratory muscles fail to perform their tasks, hypoventilation occurs and subsequently, oxygen is deficiently provided to the different tissues (including the striated muscles themselves). Therefore, aerobic metabolism becomes impaired and anaerobic pathways increase their activity, which has consequences for both energy generation and acid-base homeostasis. The latter can even become aggravated by the fact that the drop in ventilation leads to carbon dioxide retention.
The analysis of cardiac muscle dysfunction is very complex and lies beyond the scope of this review, which will focus on the functional impairment of both limb and respiratory muscles in COPD.
Chronic obstructive pulmonary disease (COPD)
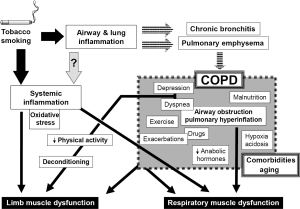
This is a highly prevalent respiratory disease, with enormous costs for both health and social care systems. The main cause of COPD is tobacco smoking, although other factors can also be involved (20,21). Inhalation of noxious particles suspended in the smoke results in airway and lung inflammation, as well as in the destruction of lung parenchyma, which are directly related to the occurrence of the two main entities included in COPD: chronic bronchitis and pulmonary emphysema. COPD has been classically defined in terms of its impact on lung function, mainly characterized by a non-fully reversible airflow obstruction (20), together with pulmonary hyperinflation and gas exchange abnormalities. Although symptoms such as coughing and breathlessness appear slowly, they progress and eventually lead to exercise limitation and death. However, in recent years it has become evident that COPD is not only a pulmonary disease since many of the symptoms are caused by the involvement of other organs and systems (22). This systemic involvement includes abnormalities in skeletal muscles, blood, nervous system and even in the bone metabolism (22-26), but its causes and mechanisms remain unclear. It is believed, however, that systemic inflammation (directly linked or not to the local inflammatory process already present in the lungs), plays a key role in the occurrence of extrapulmonary manifestations of COPD (20,22,25). Although systemic inflammatory response syndrome (SIRS) is an expression which is usually restricted to multiple organ damage and subsequent dysfunction appearing in the course of sepsis (27-29), some authors have suggested that this term or the alternative chronic systemic inflammatory syndrome (CSIS), can also be used in other entities, such as COPD (30,31), which are characterized by the presence of low level but persistent systemic inflammation and multiple organ involvement.
Muscle dysfunction in COPD
This is probably the most extensively studied systemic manifestation of COPD and can involve both respiratory and peripheral muscles (24). It is considered to be of multifactorial origin, with local and systemic factors interacting to modify, in different ways, the phenotype and function of any specific muscle (Figure 2) (32). The following pages summarize the current knowledge regarding the status of different striated muscles in COPD patients. They include structural, metabolic and functional findings as well as a review of the factors, which have been involved in muscle dysfunction.
Respiratory muscles in COPD
From the 1970s it has been well established that the function of the diaphragm deteriorates in subjects with pulmonary emphysema (33,34). This is mainly due to the dramatic increase in lung volume known as pulmonary hyperinflation (Figure 2), which shortens and flattens the diaphragm and negatively modifies its length-tension relationships. As a result, the diaphragm loses its capacity to develop contractile force (35). In addition, the involvement of the airways, inherent to COPD, implies that respiratory muscles have to cope with increased airway resistance and airflow obstruction. Both pulmonary hyperinflation and increased airway resistance increase the work of breathing, which is mainly dependent on inspiratory muscles. In other words, from a mechanical point of view, respiratory muscles need to perform heavy duty under very adverse conditions. Likewise, and from a metabolic point of view, although the nutrient and oxygen demands of respiratory muscles are relatively low under normal conditions (36), in COPD patients they become progressively higher as a consequence of their increased tasks. This is particularly important in individuals whose oxygen delivery to these muscles can be easily compromised by simultaneous gas exchange abnormalities occurring in the lungs. Therefore, respiratory muscles in COPD are also exposed to a potential metabolic imbalance between offers and demands (32).
Nevertheless, respiratory muscles are not only subject to local mechanical and metabolic factors directly deriving from changes in the airways and lung parenchyma. Like other striated muscles in the whole body they can also be influenced by systemic factors such as inflammation and oxidative stress (both of which have been detected in the blood stream of COPD patients), nutritional depletion and the effect of certain drugs used in the treatment of this condition (Figure 2) (37-42). These systemic influences will be more extensively reviewed in the following sections. Moreover, as a result of all these local and systemic factors, molecular and cellular phenomena such as focal inflammation, oxidative stress and epigenetic changes (43-45) will be present in the diaphragm and rib muscles of COPD patients.
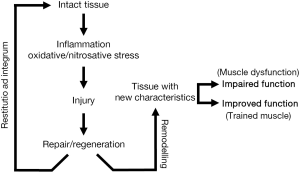
Unexpectedly and despite all these negative factors, the final result is not so negative. Certainly, the diaphragm and other respiratory muscles show an impairment in their functional properties (33,46,47). However, it has been shown that the diaphragm of COPD patients is able to develop even greater strength than that of healthy subjects when both are forced to maintain similar levels of hyperinflation (34). To explain such a paradox it is important to remember that striated muscles are very sensitive to modifications in their environment, as they are extraordinarily capable of changing their phenotype to adapt to the ongoing conditions. In keeping with this, different authors have supplied evidence that respiratory muscles actually undergo structural and metabolic changes, which would partially explain the paradox of their relatively preserved function. Phenotypic changes occurring in the respiratory muscles of COPD appear to include modifications in the expression of structural proteins such as myosin heavy chain (MyHC) isoforms, mitochondrial and capillary content, sarcomere length and fiber type proportions and sizes (48-56). Moreover, some experimental models suggest that these adaptive phenomena might be directly linked to chronic increase in respiratory loads (57), and they appear to be mediated by the occurrence of transient muscle damage (58,59), which would be followed by muscle repair/regeneration and remodeling (Figure 3) (57). However, coexisting with adaptive changes, and in addition to muscle damage, there are also indications that myopathy may be present in the respiratory muscles of COPD patients (60). Therefore, it is not so surprising that isolated fibers from their diaphragms have been shown to develop less force than those from control individuals (61).
In contrast with the relative abundance of data on inspiratory muscles, information regarding changes occurring in expiratory muscles of COPD is in short supply. This is somewhat surprising since expiratory muscle function appears to be important in COPD patients for both coughing and breathing (62-66), but it is deteriorated (67-69). This expiratory muscle dysfunction, in contrast to what occurs in inspiratory muscles, cannot be attributed to mechanical changes occurring in the lungs. In this regard, hyperinflation might even improve the length-tension relationships of abdominal expiratory muscles (70). Neither can it be ascribed to muscle deconditioning, as is considered the case for peripheral muscles (see next sections). Therefore, systemic factors leading to molecular and cellular abnormalities are the most probable culprits in expiratory muscle dysfunction occurring in COPD. From the very sparse reports available it appears that fiber phenotype is altered in these muscles (54) but their global metabolic properties appear to be maintained (71).
Peripheral muscles in COPD
This is a very heterogeneous group of contractile elements located in the upper and the lower limbs, which perform different tasks including walking and the manipulation of instruments. Although the function of both upper and lower limb muscles can be impaired in COPD patients (24,32,47,72,73), the level of dysfunction is not necessarily the same. In fact, leg muscles appear to be more severely affected than those located in the upper limbs (69,74). The reason for these differences is believed to be closely related to the differential activity of these two groups of muscles in COPD patients.
Lower limb muscle dysfunction is not merely a local problem since it has a direct impact on the exercise capacity of the patients (72,75-78). It is characterized by a reduction in both muscle strength and endurance (24,47,72,73,79), as well as an impairment in the efficiency of muscle metabolism, since lower limb muscles of COPD patients consume more oxygen for any particular workload and are characterized by an early and increased production of lactate (78). Regarding structural and metabolic findings, most of the studies have been performed in the vastus lateralis, which is a part of the quadriceps muscle. These studies have shown overall muscle mass reduction, along with smaller fibers, and a “less aerobic phenotype” (reduced percentage of oxidative fibers and MyHC-I, fewer blood vessels and capillary contacts per fiber, a reduced myoglobin content and a diminished enzyme capacity in the oxidative pathways) (53,80-89). Moreover, the fact that the oxidative capacity of the muscle is reduced, whereas the oxygen delivery is relatively preserved in COPD, supports the existence of an inefficient intracellular use of this gas (78,90). This probably explains the above mentioned early lactate production and myocyte acidification (78,87,91,92). In contrast to abnormalities in oxidative pathways, the activity of glycolytic tracks is maintained or even increased in these patients (83,87).
Although studies focused on the upper limb muscles are much less frequent than those dealing with lower extremities, the findings are consistent with the relative maintenance of histological, biochemical and functional properties in the former (69,74,93-96). Furthermore, some of these muscles show mild adaptive changes, similar to those exhibited by respiratory muscles, along with certain other modifications that are more characteristic of lower limb muscles. Deltoid muscle, for instance, shows the coexistence of different fiber size subpopulations (normal, hypertrophic and atrophic) (93), preserving the percentages of different fiber types as well as the enzyme activities in the aerobic pathways (94). The brachial biceps, on the other hand, show an unchanged fiber type composition along with a mild decrease in size as well as preserved behavior in the aerobic pathways (95,96). The final result of all of these cellular and molecular changes is a generally mild-to-moderate reduction in the functional properties of the upper limb muscles, which has a lower impact on the activities and life of the patients than lower limb muscle dysfunction (69,74).
The causes of peripheral muscle dysfunction have not been completely elucidated, but it is generally accepted that at least for the lower limbs, muscle deconditioning, resulting from a reduction in physical activity, plays a key role (Figure 2) (24,32,47). However, since most of the functional and structural limb muscle changes are only partially reversed by muscle training, other intrinsic and systemic factors are probably also implicated. Many of them would be common for all the striated muscles throughout the body.
To sum up, changes shown by different skeletal muscles in COPD patients are very heterogeneous, depending on the muscle group being judged. This indicates that these changes are most probably the result of the complex interaction of different factors, with each one being unique for any particular muscle (97). In the following paragraphs, the systemic factors and cell-molecular mechanisms that have been involved in the pathogenesis of muscle dysfunction in COPD will be briefly reviewed.
Systemic factors involved in muscle dysfunction in COPD
Inflammation
This can be considered as either a systemic or a local factor since inflammatory activity has actually been demonstrated both in different solid tissues such as skeletal muscles and in the blood of COPD patients (37,98-101). It is believed that the initial tobacco and/or other pollutants insult crosses through the alveolus-capillary interface and then immediately spreads through the systemic bloodstream targeting different organs (102). Alternatively, or complementarily, other authors sustain that the initial inflammatory process induced by these deleterious factors in the airways, lung parenchyma and pulmonary vessels is later disseminated through systemic circulation reaching different target-organs including muscles (‘spill over’ theory) (99,103-105). However, the absence of concordance between the inflammatory markers found in the blood and in other tissues, and the occasional occurrence of systemic manifestations preceding clear lung involvement strongly argues against the latter theory (42,106,107). Whether by one way or the other, it is generally accepted that chronic systemic inflammatory signal and the subsequent multi-local inflammatory activity are significant contributors to muscle dysfunction occurring in COPD (24,32,42,47). Among the evidence for the persistent systemic inflammatory signal there are studies which show increases in the serum levels of C-reactive protein (CRP), fibrinogen and different proinflammatory cytokines (37,99,101) as well as different abnormalities in circulating white cells (25,99,108). Similarly, inflammatory activity has been documented in other extrapulmonary targets such as striated muscles. In this respect, an increase in inflammatory cells has been documented within the peripheral muscles of COPD patients (100), although other authors have been unable to confirm such findings (109). In addition to cellular changes, an increase in the expression of local proinflammatory cytokines has been described in respiratory and peripheral muscles of COPD patients (100,110), although again not all the authors agree with this finding (98). It is well known that these inflammatory mediators are capable of inducing an increase in the degradation of intracellular proteins either through direct activation of proteolytic pathways or the development of oxidative stress (111,112).
One important aspect is the probable impact that exacerbations have on the levels of systemic inflammation in COPD. Some authors have demonstrated that the inflammatory load already present in the bronchial tree of the patients can be further increased by colonization or infection by microorganisms (113), which in turn may lead to exacerbations (114). This factor would support the use of local anti-inflammatory or antibacterial drugs to reduce “the inflammatory overload” present in the lungs. However, the extent to which this local ‘over inflammation’ is reflected in extrapulmonary targets such as skeletal muscles is still unclear.
Oxidative and nitrosative stress
Reactive oxygen species (ROS), a product of the oxygen metabolism, and nitric oxide (NO) are normally present in skeletal muscles. Moreover, a moderate level of ROS, which are provided by the mitochondrial respiratory chain pathway and some microsome enzymes, is necessary for excitation-contraction coupling and an appropriate muscle contraction (115). The same is true of NO (synthesized by specific enzymes in both fibers and endothelium), which plays a role in myoblastic differentiation, carbohydrate metabolism, blood flow regulation to the fibers and electromechanical muscle coupling (116,117). However, when there is an increase in the production of oxygen or nitrogen reactants and/or their scavengers are unable to buffer them, oxidative and/or nitrosative stress occur. This leads to structural damage in proteins, lipids and DNA, with important functional consequences (118-120). Interestingly, the production of free radicals is modulated by a variety of factors including the presence of inflammatory mediators, blood supply and level of activity (121,122).
Free radical stress is believed to be involved in COPD pathogenesis. Moreover, as with inflammation, oxidative and nitrosative stress appear to extend beyond the lung to reach other systems. In this respect, these phenomena have been found both in animal models and COPD patients, where they involve both respiratory and peripheral muscles (43,123,124). Moreover, the oxidative stress level within the respiratory muscles appears to be directly related to the mechanical loads they have to deal with, and directly influences their function (43). Lower limb muscles in turn show even more stress than respiratory muscles (125), and the functional consequences (126) are probably related to changes induced in key enzymes such as creatine kinase and carbonic anhydrase (127). In this case, the local oxidative stress might be caused by the reduction in muscle activity, which is known to decrease the content of reduced glutathione, while increasing both oxidized glutathione and lipid peroxidation (128). However, the presence of all these deleterious phenomena can also open new therapeutic strategies for COPD patients, such as the use of antioxidants (126,129).
Deconditioning
Deconditioning is the result of the reduction in physical activity that, as previously mentioned, is frequent in COPD patients as a consequence of their ventilatory limitation, a sedentary life style and reactive depression. The effects of deconditioning are especially evident in lower limb muscles. There is strong evidence for the key role of deconditioning in limb muscle changes since many of the structural and biochemical changes observed in COPD are similar to those induced by disuse (fibers become smaller and the proportion of type II fibers increases) (130), and are reversible with training (131). However, the fact that muscle dysfunction has been observed even in hand muscles (69), which are being continuously used even by very severe COPD patients, and that training does not completely reverse all muscle abnormalities (78,131), strongly suggests that deconditioning is not the only factor.
A particular case is the muscle dysfunction appearing in those COPD patients who have been submitted to mechanical ventilation, with or without complete sedation. This therapeutic procedure, with many different modalities, is characterized by absolute or relative muscle rest, which leads to muscle involution and dysfunction (132). However, in this specific case, disuse is not limited to limb muscles but also affects respiratory muscles (133,134). Furthermore, other factors common in critically ill patients, such as sepsis, malposition and drugs (27,134-136) can further deteriorate muscle function in COPD patients submitted to mechanical ventilation.
Nutritional abnormalities
Nutritional abnormalities expressed as body waste and changes in body composition are also frequently observed in COPD patients (40,41,137), with their prevalence dependent both on the variables analyzed and the population considered. Body mass index (BMI) is the most currently used nutritional threshold variable, since it is clearly related to life expectancy in COPD patients (138). However, this is a very general parameter that can lead to an underestimation of nutritional abnormalities, especially in women. Therefore, the fat free mass index (FFMI) has been proposed as a better and more sensitive alternative for classifying patients (139). Malnutrition associated with COPD can lead to reductions in muscle mass, changes in the proportions and size of muscle fibers (140), and muscle dysfunction (41). It has been attributed to different factors including the presence of systemic inflammation (37,42,47,101), a reduction in food intake (probably due to changes in leptin metabolism) (141), and an increase in metabolic cost derived from the increased work of breathing (which in turn is the consequence of the impairment in the mechanical properties of the ventilatory system) (42,142). The prevalence of nutritional abnormalities, however, does not appear to be homogeneous through different geographical areas, since it seems to be lower in Mediterranean countries than in Northern Europe and North America (143,144). These differences have been attributed to life-style factors such as dietary habits and the level of physical activity (145).
Gas exchange abnormalities
Ventilation-perfusion mismatching present in COPD patients frequently results in chronic hypoxia, with or without hypercapnia. In addition, respiratory muscle dysfunction can also contribute to gas exchange abnormalities through the development of absolute or relative hypoventilation. Conversely, both chronic hypoxia and hypercapnia can have effects on muscle function. Hypoxia results in a reduction of muscle strength and endurance, contributing to exercise limitation (146,147). This loss of muscle function can be explained by the induction of systemic inflammation, oxidative stress and apoptosis, the imbalance between protein synthesis and catabolism (proteostasis), the limitation in the aerobic pathways and impaired muscle regeneration (148-151). Hypercapnia, directly or through the development of respiratory acidosis, may also induce an impaired muscle proteostasis and affect muscle contractile properties (152-154).
Tobacco smoking
It is well known that even nonsymptomatic smokers can exhibit fatigability and reduced muscle resistance (155,156). This can be well explained by the anorectic effects of tobacco, which may lead to the loss of muscle mass, as well as inducing inflammation, oxidative stress, an imbalance between protein synthesis and degradation in the muscle, and blockading the neuromuscular transmission (102,106,157-159).
Drugs
Some of them, such as systemic steroids, with very well known deleterious effects on muscle structure and/or function, are used relatively frequently in the treatment of COPD patients. Steroids can induce both chronic and acute myopathies (160). In fact even low doses of these drugs can cause the chronic form, characterized by weakness of proximal muscles, if taken during a relatively long period of time (161). Acute myopathy in turn appears a few days after steroid administration, and the symptoms do not predominate in a particular muscle group. Therefore, it is not surprising that corticosteroids have always been related to muscle dysfunction in COPD patients, since they even influence their survival expectancies (161).
Anabolic hormones decrease or inefficiency
Plasma levels of testosterone, a steroid hormone with important anabolic effects such as the increase in muscle protein synthesis (162), have been shown to be reduced in some COPD patients (163,164). This abnormality has been explained by the effects of smoking, hypoxia and drug therapies (24,165) but its functional implications remain unclear since both muscle strength and endurance appear to be preserved in such patients (164). In the case of the growth hormone, another powerful anabolic agent, the problem is not the plasma level but the interaction with the insulin-like growth factor (165,166), which is altered and can potentially impair proteostasis and reduce muscle mass leading to dysfunction (42).
Exercise
This factor is essential to muscle performance but should be kept between physiological limits. When exercise is too intense it can lead to the development of metabolic dysregulations, systemic inflammation and oxidative stress, muscle damage and inhibition in the expression of genes crucial for muscle mass maintenance (59,167-171). Therefore, it is not surprising that it can also contribute to muscle dysfunction (169). Moreover, some COPD patients show marked energetic-mechanical inefficiency during exercise (78,172,173). This could be the consequence of a reduced matching between the expression of genes linked to bioenergetics and those participating in programs of muscle regeneration and remodeling (174). Furthermore, not only the intensity of the exercise but its time course can influence the response of the muscle in COPD patients, mostly in those with reduced body weight. In this respect, high intensity training programs may induce oxidative stress in the patient’s muscles during the first weeks (175) but this effect disappears if the program lasts longer (around 8 weeks) (176).
Exacerbations
The relationships between inflammation and infections present during exacerbations and muscle dysfunction have been previously analyzed. However, these acute episodes also involve other deleterious factors such as inactivity, negative energy balance and the use of systemic steroids (42,177-179). Therefore, it is not surprising that exacerbations are widely considered to be one of the factors that contribute the most to muscle wasting and dysfunction (24,32,42). Both develop early in the episode, and last for a relatively long time (42). Conversely, those patients with muscle dysfunction show an increased risk of hospital admission due to exacerbations (180,181).
Comorbidities and aging
These are also potential additional contributors to the muscle dysfunction shown by COPD patients. On the one hand, many of the most frequent comorbidities of this respiratory disorder, such as chronic heart disease, diabetes and cancer also lead to muscle wasting and dysfunction (24,32,42,140,182). On the other hand, developed societies are characterized by an increased number of elderly individuals with chronic conditions such as COPD. Aging per se associates with loss of muscle mass (sarcopenia), fibrosis, mitochondrial efficiency, functional impairment in the neuromuscular junction (183-185), and progressive muscle inability to perform daily life tasks (186).
Many of the cellular and molecular events that occur in the muscles of COPD patients have been mentioned in the previous section when reviewing the etiopathogenic factors of muscle dysfunction. This is the case of local inflammation and oxidative stress, apoptosis, muscle injury, regeneration defects, imbalance between protein synthesis and destruction, loss of capacity of enzymes in the aerobic pathways, changes in fiber size and type proportions, and findings suggesting a myopathy. These, and other findings, will be discussed in more detail in the next section.
Biological phenomena observed in muscle (Figure 4)
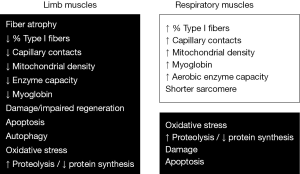
Muscle inflammation
As previously mentioned, inflammatory phenomena have been observed in some of the muscles of COPD patients. Most authors have reported increases in the number of inflammatory cells in the peripheral muscles (100,187,188), although not in the respiratory muscles of these patients (188). In contrast, there are major discrepancies among different authors with respect to the presence of inflammatory cytokines in skeletal muscles. Some of them have found increases of these substances in the patients’ peripheral muscles (100), while others have reported exactly the opposite (98,189). As for respiratory muscles, there is only one available report, and this indicates that there is an increase in different proinflammatory cytokines in this population (44).
Oxidative and nitrosative stress within the muscle
This factor is important for both its action damaging DNA, proteins and cellular lipids, and its direct impact on muscle function. Increased levels of oxidative and/or nitrosative stress have been described both in respiratory and limb muscles of COPD patients (43,123,140,190-193). This appears to be the consequence of an increased production of ROS in mitochondria (193) as well as a decrease in local antioxidants (194), although the latter appears less affected in the particular case of respiratory muscles (195). Moreover, in COPD patients the increased baseline levels of oxidative stress seem to increase even more after intense exercise (169,175). Although oxidative stress can damage different cellular structures and modify key enzymes (140,175), its role in the increase of protein destruction and fiber atrophy is not very clear (190).
Muscle damage
Although not all the authors agree (196), different signs of damage have been reported in either peripheral or respiratory muscles of COPD patients. This evidence has been observed both in the contractile structure of sarcomeres and also in the sarcolemma and other muscle structures (44,59,140,197). Interestingly, high intensity exercise appears to increase the level of muscle damage in these patients (59,198). However, as opposed to what happens in many myopathies, only small increments of fibrous and fat tissues have been observed in COPD muscles COPD (109). It should be emphasized that muscle damage is not necessarily a harmful phenomenon. As different animal models suggest, when its level is mild to moderate it can lead to muscle repair and adaptive remodeling (57,58).
Satellite cells and muscle regeneration program
These elements are closely related to the previous paragraph, since the maintenance of muscle structure depends on the balance between damage and repair/regeneration (Figure 3). Satellite cells are responsible for maintaining an adequate number of operational nuclei in muscle fibers, which in turn will favor an adequate protein synthesis and muscle mass. The number of satellite cells appears to be preserved both in the respiratory and the limb muscles of COPD patients (187,199). However, their regenerative capacity seems altered, at least in the latter, as suggested by the increase in internalized nuclei and decreased expression of late markers of regeneration (200). In keeping with this, cultured myoblasts (equivalent to satellite cells) obtained from COPD patients evidence problems in their later stages of differentiation, with difficulties in expressing adult myosins (200,201). This has been attributed to cell aging as suggested by telomere shortening (202).
Apoptosis
It must be clarified that the classical meaning of apoptosis and its histological signs is somewhat different in skeletal muscle (a syncytium) than in uninucleate cells. Indeed, here the death of one or more nuclei does not involve the death of the fiber since they can be replaced by those nuclei from satellite cells. Although the TUNEL technique has shown an increase of nuclei with ‘apoptotic signs’ in the muscles of COPD patients (either with preserved or reduced body weight) (188,203), true apoptosis has not been confirmed by electron microscopy (the gold standard technique) (188). Therefore, these signs are probably linked to the nuclear turnover, without the presence of real apoptosis.
Autophagy
This is a catabolic process involved in the elimination of excessive or altered cellular organelles. Signs of autophagy have already been found in peripheral muscles of COPD patients, with and without weight loss (140,204).
Epigenetic alterations
These are changes in the expression of certain genes without any modification in the genome. Such changes have been reported both in respiratory and peripheral muscles of patients with COPD, either with preserved or reduced body weight (45,205,206), and can be considered as a response to either chronic overload or atrophic signals, respectively.
Muscle capillarization
Although some authors have reported a decreased capillary density in the lower limb muscles of patients (80,207,208), especially in those with early occurrence of fatigue during exercise (209), other researchers have failed to confirm this finding (86,210,211). Moreover, both the external intercostal and diaphragm muscles seem to show an increased capillary density (212,213), which would be added to the other aerobic adaptations observed in these muscles. It is possible that many of these changes in the number of blood vessels are related to the level of expression of the vascular endothelial growth factor (VEGF), which is decreased in limb muscles but increased in respiratory muscles of COPD patients (98,214). Finally, animal models have shown that either emphysema or hypoxia may result in an increase in the number and length of the capillaries, as well as on their contact surface with fibers (215,216).
Mitochondrial density and function
Again a discrepancy between limb and respiratory muscles has been observed for mitochondrial density. While their number is decreased or roughly preserved in the former (quadriceps and tibialis anterior muscles, respectively) (217), it appears to be increased in the diaphragm (51). There is also an important mitochondrial dysfunction in limb muscles expressed by an uncoupling between different steps of the respiratory chain, reduced aerobic enzyme capacity, increases in phenomena linked to apoptosis and increased production of free radicals (218).
Enzymes in aerobic and anaerobic pathways
The capacity of key enzymes involved in different metabolic pathways has also been studied in detail in muscles of COPD patients. It is possible, however, that in some cases the reported changes directly depend on modifications also observed in fiber phenotype and/or the number or efficiency of some cell organelles such as mitochondrias (32). Respiratory muscles show activity increases in different enzymes participating in the oxidative pathways in COPD patients (196,219-221), while a decrease in enzyme capacity may occur in glycolytic pathways (222). On the contrary, lower limb muscles typically show less enzyme activity in their aerobic pathways, with maintenance or even increase in glycolytic enzymes (83,223,224). However, chronic respiratory failure appears to counterbalance some of these effects, since some oxidative enzymes increase their activity in those patients in such circumstances (225). Unfortunately, this would lead to metabolic uncoupling in these pathways, probably resulting in impaired muscle bioenergetics (78). Moreover, the loss of aerobic enzyme capacity can also be reverted by endurance training (131,224, 226). Finally, the upper limb and shoulder muscles roughly seem to maintain the capacity of their key enzymes (94,221,222) and can even show increases in the enzyme activity within oxidative pathways in most severe COPD patients (94).
Changes in muscle fibers
This is one of the changes consistently observed in the muscles of COPD patients. Their limb muscles show a higher proportion of type II fibers (fast-twitch contraction, predominantly anaerobic metabolism) (86,190,227), while the diaphragm and intercostal muscles seem to show changes in the opposite direction since patients increase the proportion of type I fibers (slow-twitch contraction, aerobic metabolism and fatigue resistant) (48,61,228). All these changes depend on parallel modifications in the expression of adult myosin isoforms in response to those stimuli mentioned in preceding sections (with an apparent key role for the level of activity of each muscle) (48,49,229). It is worth noting that the proportion of slow-twitch fibers has recently been related to mortality in COPD patients (230). Furthermore, it seems clear that lower limb muscle fibers disclose a reduced size in those patients with loss of body weight (86,190,231). This atrophy is especially evident for type II fibers (190,231). As already mentioned, the situation is different in the muscles of the upper extremities, as they appear to keep their fiber cross sectional area (93,232). There is more discrepancy regarding the size of diaphragmatic fibers. Some authors have reported atrophy (233,234), while others have not been able to find such an abnormality in COPD patients (45,188,235). As regards to the function of the fibers, it seems to be altered in respiratory muscles (236,237) but surprisingly, not in limb muscles (238) of patients. In this regard, the diaphragm (237) as well as the intercostal muscle (236) show a decline in the strength of their fibers (normalized by size).
Protein synthesis and degradation
The imbalance between protein synthesis and breakdown appears as the key mechanism for the loss of muscle mass and function (42). Indeed, muscle mass is dynamically maintained by the balance between these two processes. When such a balance breaks and destruction prevails, muscle mass is reduced and that has an important impact on muscle function. It is well known that protein synthesis is reduced in underweight emphysema patients (239), but not in those with preserved body weight (240). Protein synthesis depends on the availability of substrates and the activity of signaling pathways. Regarding the former, several authors have reported that there is a reduction in plasma levels of glutamine, glutamate and alanine, as well as in some branched-chain amino acids (such as leucine) in COPD patients with low weight (239,241-243). The results for other amino acids are much more controversial (244). With regards to signaling pathways, the protein kinases B (Akt) and rapamycin (mTOR), which are activated in response to the input of nutrients and anabolic hormones, play a determinant role in muscle protein synthesis. Some authors have observed a decrease in the expression of Akt in limb muscles of COPD patients (204), while others have not confirmed these changes (206). Moreover, it has been reported that in COPD patients with severe hypoxemia, high intensity exercise reduces phosphorylation of Akt, potentially contributing to decreased protein synthesis (245). Among the factors that may explain the deficits in the synthesis of proteins in COPD patients with low body weight are the changes in anabolic hormones or their signaling pathways, and the presence of systemic inflammation and oxidative stress (246).
Protein breakdown in turn can occur through different pathways, including that of the proteasome. This pathway requires previous ubiquitination of the target protein, which needs the intervention of atrogenin-1 and MuRF1, both controlled by FoxO transcription factors and their regulators (247,248). These factors seem to be overexpressed in skeletal muscles of COPD patients (238,249,250). Moreover, protein ubiquitination has already been demonstrated in both limb muscles (140) and diaphragm (251) of such patients. In a second pathway, proteins can be degraded by the lysosomal enzyme system, which includes lipases, glycosidases and cathepsins, among others. This system is closely related with the aforementioned cell phenomenon of autophagy (252), which increases in the muscles of COPD patients (204), although some authors have only been able to find it in those patients associating low weight (140). The third catabolic pathway is that of calpains, not lysosomal proteases that are highly dependent on calcium concentration. To date no studies have clarified the role of this proteolytic system in COPD patient muscles. Finally, there is also the pathway of caspases, closely linked to apoptosis. This can be activated by different factors, including exercise (253). Unfortunately, to date the results regarding its role in COPD are controversial. Some authors have found no changes in the levels of these enzymes in either peripheral or respiratory muscles of those patients with preserved body weight (188), while others have reported an increase in caspase 3 activity in the diaphragm of these patients (251). Moreover, the activity of caspases is still unknown in the muscles of patients with weight loss or following high intensity exercise. As with reduced protein synthesis, the activation of their degradation may be due to different factors present in COPD including tobacco smoking, exacerbations, inflammation, oxidative stress and treatment with steroids (246).
Signs of true myopathy
For a long time there was debate as to whether muscle abnormalities associated with COPD constitute a real myopathy (254). Strictly speaking, the definition of myopathy needs a number of specific muscle findings such as necrosis, inflammatory infiltrates, immune phenomena and/or inclusion bodies. Of all of them, only paracrystalline inclusions have been reported in the diaphragm of one isolated patient (60). Therefore, in recent years there is a strong tendency to consider that COPD muscle abnormalities do not constitute a true myopathy. The only exception is the myopathic alteration secondary to treatments with systemic steroids.
Conclusions
Taking everything into account, it can be stated that skeletal muscles show structural and functional changes in COPD patients, and that these changes are the result of the complex interaction of multiple factors, which is specific to each particular muscle. Tobacco, systemic inflammation and nutritional abnormalities seem to be important for all the different muscle groups, whereas geometrical changes occurring in the thorax are specifically harmful for respiratory muscles, and deconditioning is more detrimental for peripheral muscles.
Acknowledgements
The authors would like to thank R. Marshall and J. McFarland for their assistance with editing.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Carroll SB. Genetics and the making of Homo sapiens. Nature 2003;422:849-57. [PubMed]
- Kibler WB. Normal shoulder mechanics and function. Instr Course Lect 1997;46:39-42. [PubMed]
- Vaughan CL. Theories of bipedal walking: an odyssey. J Biomech 2003;36:513-23. [PubMed]
- Edwards RHT, Faulkner JA. Structure and function of the respiratory muscles. In: Roussos C, editor. The Thorax. New York: Marcel Dekker Inc., 1995:185-217.
- Macklem PT. The act of breathing. In: Roussos C, editor. The thorax. New York: Marcel Dekker Inc., 1995:445-56.
- Derenne JP, Macklem PT, Roussos C. The respiratory muscles: mechanics, control, and pathophysiology. Am Rev Respir Dis 1978;118:119-33. [PubMed]
- De Troyer A, Farkas G. Mechanics of the parasternal intercostals in prone dogs: statics and dynamics. J Appl Physiol (1985) 1993;74:2757-62. [PubMed]
- De Troyer A. Interaction between the canine diaphragm and intercostal muscles in lung expansion. J J Appl Physiol (1985) 2005;98:795-803. [PubMed]
- De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev 2005;85:717-56. [PubMed]
- De Troyer A, Leduc D. Effect of diaphragmatic contraction on the action of the canine parasternal intercostals. J Appl Physiol (1985) 2006;101:169-75. [PubMed]
- Hug F, Raux M, Prella M, et al. Optimized analysis of surface electromyograms of the scalenes during quiet breathing in humans. Respir Physiol Neurobiol 2006;150:75-81. [PubMed]
- Legrand A, Schneider E, Gevenois PA, et al. Respiratory effects of the scalene and sternomastoid muscles in humans. J Appl Physiol (1985) 2003;94:1467-72. [PubMed]
- Decramer M. Hyperinflation and respiratory muscle interaction. Eur Respir J 1997;10:934-41. [PubMed]
- Orozco-Levi M, Gea J, Monells J, et al. Activity of latissimus dorsi muscle during inspiratory threshold loads. Eur Respir J 1995;8:441-5. [PubMed]
- Bellemare F, Bono D, D’Angelo E. Electrical and mechanical output of the expiratory muscles in anesthetized dogs. Respir Physiol 1991;84:171-83. [PubMed]
- Chang AB. The physiology of cough. Paediatr Respir Rev 2006;7:2-8. [PubMed]
- Fuller D, Sullivan J, Fregosi RF. Expiratory muscle endurance performance after exhaustive submaximal exercise. J Appl Physiol (1985) 1996;80:1495-502. [PubMed]
- Epstein SK. An overview of respiratory muscle function. Clin Chest Med 1994;15:619-39. [PubMed]
- Gea J, Barreiro E, Orozco-Levi M. Skeletal muscle adaptation to disease states. In: Bottinelli R, Reggiani C, editors. Skeletal Muscle Plasticity in Health and Disease: From genes to whole muscle. Dordrecht: Springer, 2006:315-60.
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. Accessed July 2015. Available online: http://www.goldcopd.org/
- Orozco-Levi M, Garcia-Aymerich J, Villar J, et al. Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J 2006;27:542-6. [PubMed]
- Evans RA, Morgan MD. The systemic nature of chronic lung disease. Clin Chest Med 2014;35:283-93. [PubMed]
- Freixa X, Portillo K, Paré C, et al. Echocardiographic abnormalities in patients with COPD at their first hospital admission. Eur Respir J 2013;41:784-91. [PubMed]
- Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014;189:e15-62. [PubMed]
- Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med 2014;35:71-86. [PubMed]
- Romme EA, Smeenk FW, Rutten EP, et al. Osteoporosis in chronic obstructive pulmonary disease. Expert Rev Respir Med 2013;7:397-410. [PubMed]
- Bosmann M, Ward PA. The inflammatory response in sepsis. Trends Immunol 2013;34:129-36. [PubMed]
- Maloney PJ. Sepsis and septic shock. Emerg Med Clin North Am 2013;31:583-600. [PubMed]
- de Jong HK, van der Poll T, Wiersinga WJ. The systemic pro-inflammatory response in sepsis. J Innate Immun 2010;2:422-30. [PubMed]
- Wouters E. COPD: from obstructive lung disease to chronic systemic inflammatory syndrome? Pneumologie 2009;63 Suppl 2:S107-12. [PubMed]
- Kirdar S, Serter M, Ceylan E, et al. Adiponectin as a biomarker of systemic inflammatory response in smoker patients with stable and exacerbation phases of chronic obstructive pulmonary disease. Scand J Clin Lab Invest 2009;69:219-24. [PubMed]
- Barreiro E, Gea J. Respiratory and Limb Muscle Dysfunction in COPD. COPD 2015;12:413-26. [PubMed]
- Rochester DF, Braun NM, Arora NS. Respiratory muscle strength in chronic obstructive pulmonary disease. Am Rev Respir Dis 1979;119:151-4. [PubMed]
- Similowski T, Yan S, Gauthier AP, et al. Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med 1991;325:917-23. [PubMed]
- Goldman MD, Grassino A, Mead J, et al. Mechanics of the human diaphragm during voluntary contraction: dynamics. J Appl Physiol Respir Environ Exerc Physiol 1978;44:840-8. [PubMed]
- Westerbald H, Lännergren J, Allen DG. Fatigue of striated muscles: Metabolic aspects. Chapter 6. In: Roussos C, editor. The Thorax. New York: Marcel Dekker Inc., 1995:219-34.
- Di Francia M, Barbier D, Mege JL, et al. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994;150:1453-5. [PubMed]
- Rahman I, Morrison D, Donaldson K, et al. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 1996;154:1055-60. [PubMed]
- Agustí A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One 2012;7:e37483. [PubMed]
- Schols AM. Nutrition in chronic obstructive pulmonary disease. Curr Opin Pulm Med 2000;6:110-5. [PubMed]
- Remels AH, Gosker HR, Langen RC, et al. The mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol (1985) 2013;114:1253-62. [PubMed]
- Gea J, Agustí A, Roca J. Pathophysiology of muscle dysfunction in COPD. J Appl Physiol (1985) 2013;114:1222-34. [PubMed]
- Barreiro E, de la Puente B, Minguella J, et al. Oxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;171:1116-24. [PubMed]
- Casadevall C, Coronell C, Ramírez-Sarmiento AL, et al. Upregulation of pro-inflammatory cytokines in the intercostal muscles of COPD patients. Eur Respir J 2007;30:701-7. [PubMed]
- Puig-Vilanova E, Aguiló R, Rodríguez-Fuster A, et al. Epigenetic mechanisms in respiratory muscle dysfunction of patients with chronic obstructive pulmonary disease. PLoS One 2014;9:e111514. [PubMed]
- Bellemare F, Grassino A. Force reserve of the diaphragm in patients with chronic obstructive pulmonary disease. J Appl Physiol Respir Environ Exerc Physiol 1983;55:8-15. [PubMed]
- Barreiro E, Bustamante V, Cejudo P, et al. Guidelines for the Evaluation and Treatment of Muscle Dysfunction in Patients With Chronic Obstructive Pulmonary Disease. Arch Bronconeumol 2015;51:384-95. [PubMed]
- Levine S, Kaiser L, Leferovich J, et al. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med 1997;337:1799-806. [PubMed]
- Gea JG. Myosin gene expression in the respiratory muscles. Eur Respir J 1997;10:2404-10. [PubMed]
- Clanton TL, Levine S. Respiratory muscle fiber remodeling in chronic hyperinflation: dysfunction or adaptation? J Appl Physiol (1985) 2009;107:324-35. [PubMed]
- Orozco-Levi M, Gea J, Lloreta JL, et al. Subcellular adaptation of the human diaphragm in chronic obstructive pulmonary disease. Eur Respir J 1999;13:371-8. [PubMed]
- Campbell JA, Hughes RL, Sahgal V, et al. Alterations in intercostal muscle morphology and biochemistry in patients with obstructive lung disease. Am Rev Respir Dis 1980;122:679-86. [PubMed]
- Caron MA, Debigaré R, Dekhuijzen PN, et al. Comparative assessment of the quadriceps and the diaphragm in patients with COPD. J Appl Physiol (1985) 2009;107:952-61. [PubMed]
- Sanchez J, Derenne JP, Debesse B, et al. Typology of the respiratory muscles in normal men and in patients with moderate chronic respiratory diseases. Bull Eur Physiopathol Respir 1982;18:901-14. [PubMed]
- Hards JM, Reid WD, Pardy RL, et al. Respiratory muscle fiber morphometry. Correlation with pulmonary function and nutrition. Chest 1990;97:1037-44. [PubMed]
- Levine S, Nguyen T, Friscia M, et al. Parasternal intercostal muscle remodeling in severe chronic obstructive pulmonary disease. J Appl Physiol (1985) 2006;101:1297-302. [PubMed]
- Gea J, Hamid Q, Czaika G, et al. Expression of myosin heavy-chain isoforms in the respiratory muscles following inspiratory resistive breathing. Am J Respir Crit Care Med 2000;161:1274-8. [PubMed]
- Zhu E, Petrof BJ, Gea J, et al. Diaphragm muscle fiber injury after inspiratory resistive breathing. Am J Respir Crit Care Med 1997;155:1110-6. [PubMed]
- Orozco-Levi M, Lloreta J, Minguella J, et al. Injury of the human diaphragm associated with exertion and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1734-9. [PubMed]
- Lloreta J, Orozco M, Gea J, et al. Selective diaphragmatic mitochondrial abnormalities in a patient with marked air flow obstruction. Ultrastruct Pathol 1996;20:67-71. [PubMed]
- Levine S, Nguyen T, Kaiser LR, et al. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am J Respir Crit Care Med 2003;168:706-13. [PubMed]
- Dodd DS, Brancatisano T, Engel LA. Chest wall mechanics during exercise in patients with severe chronic air-flow obstruction. Am Rev Respir Dis 1984;129:33-8. [PubMed]
- Ninane V, Rypens F, Yernault JC, et al. Abdominal muscle use during breathing in patients with chronic airflow obstruction. Am Rev Respir Dis 1992;146:16-21. [PubMed]
- Ninane V, Yernault JC, de Troyer A. Intrinsic PEEP in patients with chronic obstructive pulmonary disease. Role of expiratory muscles. Am Rev Respir Dis 1993;148:1037-42. [PubMed]
- O’Donnell DE, Sanii R, Anthonisen NR, et al. Expiratory resistive loading in patients with severe chronic air-flow limitation. An evaluation of ventilatory mechanics and compensatory responses. Am Rev Respir Dis 1987;136:102-7. [PubMed]
- Gorini M, Misuri G, Duranti R, et al. Abdominal muscle recruitment and PEEPi during bronchoconstriction in chronic obstructive pulmonary disease. Thorax 1997;52:355-61. [PubMed]
- Mota S, Güell R, Barreiro E, et al. Relationship between expiratory muscle dysfunction and dynamic hyperinflation in advanced chronic obstructive pulmonary disease. Arch Bronconeumol 2009;45:487-95. [PubMed]
- Ramírez-Sarmiento A, Orozco-Levi M, Barreiro E, et al. Expiratory muscle endurance in chronic obstructive pulmonary disease. Thorax 2002;57:132-6. [PubMed]
- Gosselink R, Troosters T, Decramer M. Distribution of muscle weakness in patients with stable chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2000;20:353-60. [PubMed]
- Arnold JS, Thomas AJ, Kelsen SG. Length-tension relationship of abdominal expiratory muscles: effect of emphysema. J Appl Physiol (1985) 1987;62:739-45. [PubMed]
- Ferrer A, Orozco-Levi M, Gea J, et al. Mechanical and metabolic reproducibility of resistance test of expiratory muscles with incremental threshold loading. Arch Bronconeumol 2000;36:303-12. [PubMed]
- Hamilton AL, Killian KJ, Summers E, et al. Muscle strength, symptom intensity, and exercise capacity in patients with cardiorespiratory disorders. Am J Respir Crit Care Med 1995;152:2021-31. [PubMed]
- Coronell C, Orozco-Levi M, Méndez R, et al. Relevance of assessing quadriceps endurance in patients with COPD. Eur Respir J 2004;24:129-36. [PubMed]
- Castagna O, Boussuges A, Vallier JM, et al. Is impairment similar between arm and leg cranking exercise in COPD patients? Respir Med 2007;101:547-53. [PubMed]
- Killian KJ, Leblanc P, Martin DH, et al. Exercise capacity and ventilatory, circulatory, and symptom limitation in patients with chronic airflow limitation. Am Rev Respir Dis 1992;146:935-40. [PubMed]
- Killian KJ, Summers E, Jones NL, et al. Dyspnea and leg effort during incremental cycle ergometry. Am Rev Respir Dis 1992;145:1339-45. [PubMed]
- Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015;70:213-8. [PubMed]
- Sala E, Roca J, Marrades RM, et al. Effects of endurance training on skeletal muscle bioenergetics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:1726-34. [PubMed]
- Evans RA, Kaplovitch E, Beauchamp MK, et al. Is quadriceps endurance reduced in COPD?: a systematic review. Chest 2015;147:673-84. [PubMed]
- Eliason G, Abdel-Halim SM, Piehl-Aulin K, et al. Alterations in the muscle-to-capillary interface in patients with different degrees of chronic obstructive pulmonary disease. Respir Res 2010;11:97. [PubMed]
- Hildebrand IL, Sylvén C, Esbjörnsson M, et al. Does chronic hypoxaemia induce transformations of fibre types? Acta Physiol Scand 1991;141:435-9. [PubMed]
- Jakobsson P, Jorfeldt L, Brundin A. Skeletal muscle metabolites and fibre types in patients with advanced chronic obstructive pulmonary disease (COPD), with and without chronic respiratory failure. Eur Respir J 1990;3:192-6. [PubMed]
- Jakobsson P, Jorfeldt L, Henriksson J. Metabolic enzyme activity in the quadriceps femoris muscle in patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995;151:374-7. [PubMed]
- Simard C, Maltais F, Leblanc P, et al. Mitochondrial and capillarity changes in vastus lateralis muscle of COPD patients: electron microscopy study. Med Sci Sports Exerc 1996;28:S95.
- Satta A, Migliori GB, Spanevello A, et al. Fibre types in skeletal muscles of chronic obstructive pulmonary disease patients related to respiratory function and exercise tolerance. Eur Respir J 1997;10:2853-60. [PubMed]
- Whittom F, Jobin J, Simard PM, et al. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc 1998;30:1467-74. [PubMed]
- Maltais F, Simard AA, Simard C, et al. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med 1996;153:288-93. [PubMed]
- Wuyam B, Payen JF, Levy P, et al. Metabolism and aerobic capacity of skeletal muscle in chronic respiratory failure related to chronic obstructive pulmonary disease. Eur Respir J 1992;5:157-62. [PubMed]
- Natanek SA, Gosker HR, Slot IG, et al. Pathways associated with reduced quadriceps oxidative fibres and endurance in COPD. Eur Respir J 2013;41:1275-83. [PubMed]
- Maltais F, Jobin J, Sullivan MJ, et al. Lower limb metabolic and hemodynamic responses during exercise in normal subjects and in COPD. J Appl Physiol 1998;84:1573-80. [PubMed]
- Engelen MP, Schols AM, Does JD, et al. Exercise-induced lactate increase in relation to muscle substrates in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:1697-704. [PubMed]
- Casaburi R, Patessio A, Ioli F, et al. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis 1991;143:9-18. [PubMed]
- Hernández N, Orozco-Levi M, Belalcázar V, et al. Dual morphometrical changes of the deltoid muscle in patients with COPD. Respir Physiol Neurobiol 2003;134:219-29. [PubMed]
- Gea JG, Pasto M, Carmona MA, et al. Metabolic characteristics of the deltoid muscle in patients with chronic obstructive pulmonary disease. Eur Respir J 2001;17:939-45. [PubMed]
- Sato Y, Asoh T, Honda Y, et al. Morphologic and histochemical evaluation of muscle in patients with chronic pulmonary emphysema manifesting generalized emaciation. Eur Neurol 1997;37:116-21. [PubMed]
- Shields GS, Coissi GS, Jimenez-Royo P, et al. Bioenergetics and intermuscular fat in chronic obstructive pulmonary disease-associated quadriceps weakness. Muscle Nerve 2015;51:214-21. [PubMed]
- Gea J, Orozco-Levi M, Barreiro E, et al. Structural and functional changes in the skeletal muscles of COPD patients: the "compartments" theory. Monaldi Arch Chest Dis 2001;56:214-24. [PubMed]
- Barreiro E, Schols AM, Polkey MI, et al. Cytokine profile in quadriceps muscles of patients with severe COPD. Thorax 2008;63:100-7. [PubMed]
- Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004;59:574-80. [PubMed]
- Montes de Oca M, Torres SH, De Sanctis J, et al. Skeletal muscle inflammation and nitric oxide in patients with COPD. Eur Respir J 2005;26:390-7. [PubMed]
- Schols AM, Buurman WA, Staal van den Brekel AJ, et al. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax 1996;51:819-24. [PubMed]
- Zhang J, Liu Y, Shi J, et al. Side-stream cigarette smoke induces dose-response in systemic inflammatory cytokine production and oxidative stress. Exp Biol Med (Maywood) 2002;227:823-9. [PubMed]
- Salvi S, Blomberg A, Rudell B, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med 1999;159:702-9. [PubMed]
- van Eeden SF, Tan WC, Suwa T, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am J Respir Crit Care Med 2001;164:826-30. [PubMed]
- Nordskog BK, Fields WR, Hellmann GM. Kinetic analysis of cytokine response to cigarette smoke condensate by human endothelial and monocytic cells. Toxicology 2005;212:87-97. [PubMed]
- Barreiro E, Peinado VI, Galdiz JB, et al. Cigarette smoke-induced oxidative stress: A role in chronic obstructive pulmonary disease skeletal muscle dysfunction. Am J Respir Crit Care Med 2010;182:477-88. [PubMed]
- Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of ‘overspill’ of inflammatory mediators from the lungs? Review of the evidence. Thorax 2010;65:930-6. [PubMed]
- Sauleda J, García-Palmer FJ, González G, et al. The activity of cytochrome oxidase is increased in circulating lymphocytes of patients with chronic obstructive pulmonary disease, asthma, and chronic arthritis. Am J Respir Crit Care Med 2000;161:32-5. [PubMed]
- Gosker HR, Kubat B, Schaart G, et al. Myopathological features in skeletal muscle of patients with chronic obstructive pulmonary disease. Eur Respir J 2003;22:280-5. [PubMed]
- Rabinovich RA, Figueras M, Ardite E, et al. Increased tumour necrosis factor-alpha plasma levels during moderate-intensity exercise in COPD patients. Eur Respir J 2003;21:789-94. [PubMed]
- Flores EA, Bistrian BR, Pomposelli JJ, et al. Infusion of tumor necrosis factor/cachectin promotes muscle catabolism in the rat. A synergistic effect with interleukin 1. J Clin Invest 1989;83:1614-22. [PubMed]
- Hall-Angerås M, Angerås U, Zamir O, et al. Interaction between corticosterone and tumor necrosis factor stimulated protein breakdown in rat skeletal muscle, similar to sepsis. Surgery 1990;108:460-6. [PubMed]
- Hill AT, Campbell EJ, Hill SL, et al. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med 2000;109:288-95. [PubMed]
- White AJ, Gompertz S, Stockley RA. Chronic obstructive pulmonary disease . 6: The aetiology of exacerbations of chronic obstructive pulmonary disease. Thorax 2003;58:73-80. [PubMed]
- Reid MB, Haack KE, Franchek KM, et al. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol (1985) 1992;73:1797-804. [PubMed]
- Kobzik L, Reid MB, Bredt DS, et al. Nitric oxide in skeletal muscle. Nature 1994;372:546-8. [PubMed]
- Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 2001;81:209-237. [PubMed]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 1996;271:C1424-37. [PubMed]
- Tamir S, Burney S, Tannenbaum SR. DNA damage by nitric oxide. Chem Res Toxicol 1996;9:821-7. [PubMed]
- Eiserich JP, Estévez AG, Bamberg TV, et al. Microtubule dysfunction by posttranslational nitrotyrosination of alpha-tubulin: a nitric oxide-dependent mechanism of cellular injury. Proc Natl Acad Sci U S A 1999;96:6365-70. [PubMed]
- Supinski G. Free radical induced respiratory muscle dysfunction. Mol Cell Biochem 1998;179:99-110. [PubMed]
- Parks DA, Granger DN. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Physiol 1983;245:G285-9. [PubMed]
- Barreiro E, Gea J, Corominas JM, et al. Nitric oxide synthases and protein oxidation in the quadriceps femoris of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2003;29:771-8. [PubMed]
- Gosker HR, Bast A, Haenen GR, et al. Altered antioxidant status in peripheral skeletal muscle of patients with COPD. Respir Med 2005;99:118-25. [PubMed]
- Gea J, Barreiro E, Hussain SNA. Differential oxidative stress and nitrosative stress profiles in respiratory and peripheral muscles of COPD patients. Proc Am Thorac Soc 2006;3:A26.
- Koechlin C, Couillard A, Simar D, et al. Does oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease? Am J Respir Crit Care Med 2004;169:1022-7. [PubMed]
- Barreiro E, Gea J, Matar G, et al. Expression and carbonylation of creatine kinase in the quadriceps femoris muscles of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2005;33:636-42. [PubMed]
- Kondo H, Miura M, Nakagaki I, et al. Trace element movement and oxidative stress in skeletal muscle atrophied by immobilization. Am J Physiol 1992;262:E583-90. [PubMed]
- Barreiro E, Gáldiz JB, Mariñán M, et al. Respiratory loading intensity and diaphragm oxidative stress: N-acetyl-cysteine effects. J Appl Physiol (1985) 2006;100:555-63. [PubMed]
- Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc 1997;29:197-206. [PubMed]
- Maltais F, LeBlanc P, Simard C, et al. Skeletal muscle adaptation to endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;154:442-7. [PubMed]
- Winkelman C. Inactivity and inflammation in the critically ill patient. Crit Care Clin 2007;23:21-34. [PubMed]
- Le Bourdelles G, Viires N, Boczkowski J, et al. Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am J Respir Crit Care Med 1994;149:1539-44. [PubMed]
- Díaz MC, Ospina-Tascón GA, Salazar C BC. Respiratory muscle dysfunction: a multicausal entity in the critically ill patient undergoing mechanical ventilation. Arch Bronconeumol 2014;50:73-7. [PubMed]
- Deconinck N, Van Parijs V, Beckers-Bleukx G, et al. Critical illness myopathy unrelated to corticosteroids or neuromuscular blocking agents. Neuromuscul Disord 1998;8:186-92. [PubMed]
- Polkey MI, Moxham J. Clinical aspects of respiratory muscle dysfunction in the critically ill. Chest 2001;119:926-39. [PubMed]
- Schols AM, Ferreira IM, Franssen FM, et al. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respir J 2014;44:1504-20. [PubMed]
- Schols AM, Slangen J, Volovics L, et al. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1791-7. [PubMed]
- Schols AM, Broekhuizen R, Weling-Scheepers CA, et al. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005;82:53-9. [PubMed]
- Puig-Vilanova E, Rodriguez DA, Lloreta J, et al. Oxidative stress, redox signaling pathways, and autophagy in cachectic muscles of male patients with advanced COPD and lung cancer. Free Radic Biol Med 2015;79:91-108. [PubMed]
- Schols AM, Creutzberg EC, Buurman WA, et al. Plasma leptin is related to proinflammatory status and dietary intake in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:1220-6. [PubMed]
- Guenette JA, Chin RC, Cheng S, et al. Mechanisms of exercise intolerance in global initiative for chronic obstructive lung disease grade 1 COPD. Eur Respir J 2014;44:1177-87. [PubMed]
- Garcia-Aymerich J, Gómez FP, Benet M, et al. Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes. Thorax 2011;66:430-7. [PubMed]
- Coronell C, Orozco-Levi M, Gea J. COPD and body weight in a Mediterranean population. Clin Nutr 2002;21:437-author reply 437-8. [PubMed]
- Gea J, Martínez-Llorens J, Barreiro E. Nutritional abnormalities in chronic obstructive pulmonary disease. Med Clin (Barc) 2014;143:78-84. [PubMed]
- Deboeck G, Moraine JJ, Naeije R. Respiratory muscle strength may explain hypoxia-induced decrease in vital capacity. Med Sci Sports Exerc 2005;37:754-8. [PubMed]
- Romer LM, Haverkamp HC, Amann M, et al. Effect of acute severe hypoxia on peripheral fatigue and endurance capacity in healthy humans. Am J Physiol Regul Integr Comp Physiol 2007;292:R598-606. [PubMed]
- Pastoris O, Dossena M, Foppa P, et al. Modifications by chronic intermittent hypoxia and drug treatment on skeletal muscle metabolism. Neurochem Res 1995;20:143-50. [PubMed]
- Brunelle JK, Chandel NS. Oxygen deprivation induced cell death: an update. Apoptosis 2002;7:475-82. [PubMed]
- Gonzalez NC, Wood JG. Alveolar hypoxia-induced systemic inflammation: what low PO(2) does and does not do. Adv Exp Med Biol 2010;662:27-32. [PubMed]
- Yun Z, Lin Q, Giaccia AJ. Adaptive myogenesis under hypoxia. Mol Cell Biol 2005;25:3040-55. [PubMed]
- Aguar MC, Gea J, Aran X, et al. Modificaciones de la actividad mecánica del diafragma inducidas por la inhalación de CO2 en pacientes con EPOC. Arch Bronconeumol 1993;29:226-8.
- Rafferty GF, Lou Harris M, Polkey MI, et al. Effect of hypercapnia on maximal voluntary ventilation and diaphragm fatigue in normal humans. Am J Respir Crit Care Med 1999;160:1567-71. [PubMed]
- England BK, Chastain JL, Mitch WE. Abnormalities in protein synthesis and degradation induced by extracellular pH in BC3H1 myocytes. Am J Physiol 1991;260:C277-82. [PubMed]
- Corwin EJ, Klein LC, Rickelman K. Predictors of fatigue in healthy young adults: moderating effects of cigarette smoking and gender. Biol Res Nurs 2002;3:222-33. [PubMed]
- Morse CI, Wüst RC, Jones DA, et al. Muscle fatigue resistance during stimulated contractions is reduced in young male smokers. Acta Physiol (Oxf) 2007;191:123-9. [PubMed]
- Wüst RC, Morse CI, de Haan A, et al. Skeletal muscle properties and fatigue resistance in relation to smoking history. Eur J Appl Physiol 2008;104:103-10. [PubMed]
- Rom O, Kaisari S, Aizenbud D, et al. Sarcopenia and smoking: a possible cellular model of cigarette smoke effects on muscle protein breakdown. Ann N Y Acad Sci 2012;1259:47-53. [PubMed]
- Petersen AM, Magkos F, Atherton P, et al. Smoking impairs muscle protein synthesis and increases the expression of myostatin and MAFbx in muscle. Am J Physiol Endocrinol Metab 2007;293:E843-8. [PubMed]
- Goldberg AL, Goodman HM. Relationship between cortisone and muscle work in determining muscle size. J Physiol 1969;200:667-75. [PubMed]
- Decramer M, de Bock V, Dom R. Functional and histologic picture of steroid-induced myopathy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;153:1958-64. [PubMed]
- Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men--a clinical research center study. J Clin Endocrinol Metab 1996;81:3469-75. [PubMed]
- Karadag F, Ozcan H, Karul AB, et al. Sex hormone alterations and systemic inflammation in chronic obstructive pulmonary disease. Int J Clin Pract 2009;63:275-81. [PubMed]
- Laghi F, Langbein WE, Antonescu-Turcu A, et al. Respiratory and skeletal muscles in hypogonadal men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;171:598-605. [PubMed]
- Creutzberg EC, Casaburi R. Endocrinological disturbances in chronic obstructive pulmonary disease. Eur Respir J Suppl 2003;46:76s-80s. [PubMed]
- Scalvini S, Volterrani M, Vitacca M, et al. Plasma hormone levels and haemodynamics in patients with chronic obstructive lung disease. Monaldi Arch Chest Dis 1996;51:380-6. [PubMed]
- Franssen FM, Sauerwein HP, Ackermans MT, et al. Increased postabsorptive and exercise-induced whole-body glucose production in patients with chronic obstructive pulmonary disease. Metabolism 2011;60:957-64. [PubMed]
- Couillard A, Koechlin C, Cristol JP, et al. Evidence of local exercise-induced systemic oxidative stress in chronic obstructive pulmonary disease patients. Eur Respir J 2002;20:1123-9. [PubMed]
- Couillard A, Maltais F, Saey D, et al. Exercise-induced quadriceps oxidative stress and peripheral muscle dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:1664-9. [PubMed]
- Mercken EM, Hageman GJ, Langen RC, et al. Decreased exercise-induced expression of nuclear factor-κB-regulated genes in muscle of patients with COPD. Chest 2011;139:337-46. [PubMed]
- Van Helvoort HA, Heijdra YF, Thijs HM, et al. Exercise-induced systemic effects in muscle-wasted patients with COPD. Med Sci Sports Exerc 2006;38:1543-52. [PubMed]
- Layec G, Haseler LJ, Richardson RS, et al. The effect of higher ATP cost of contraction on the metabolic response to graded exercise in patients with chronic obstructive pulmonary disease. J Appl Physiol (1985) 2012;112:1041-8. [PubMed]
- Richardson RS, Leek BT, Gavin TP, et al. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak VO2 with small muscle mass exercise. Am J Respir Crit Care Med 2004;169:89-96. [PubMed]
- Turan N, Kalko S, Stincone A, et al. A systems biology approach identifies molecular networks defining skeletal muscle abnormalities in chronic obstructive pulmonary disease. PLoS Comput Biol 2011;7:e1002129. [PubMed]
- Barreiro E, Rabinovich R, Marin-Corral J, et al. Chronic endurance exercise induces quadriceps nitrosative stress in patients with severe COPD. Thorax 2009;64:13-9. [PubMed]
- Rodriguez DA, Kalko S, Puig-Vilanova E, et al. Muscle and blood redox status after exercise training in severe COPD patients. Free Radic Biol Med 2012;52:88-94. [PubMed]
- Abdellaoui A, Préfaut C, Gouzi F, et al. Skeletal muscle effects of electrostimulation after COPD exacerbation: a pilot study. Eur Respir J 2011;38:781-8. [PubMed]
- Vermeeren MA, Schols AM, Wouters EF. Effects of an acute exacerbation on nutritional and metabolic profile of patients with COPD. Eur Respir J 1997;10:2264-9. [PubMed]
- Pitta F, Troosters T, Probst VS, et al. Physical activity and hospitalization for exacerbation of COPD. Chest 2006;129:536-44. [PubMed]
- Ansari K, Keaney N, Taylor I, et al. Muscle weakness, health status and frequency of exacerbations in chronic obstructive pulmonary disease. Postgrad Med J 2012;88:372-6. [PubMed]
- Vilaró J, Ramirez-Sarmiento A, Martínez-Llorens JM, et al. Global muscle dysfunction as a risk factor of readmission to hospital due to COPD exacerbations. Respir Med 2010;104:1896-902. [PubMed]
- Rehn TA, Munkvik M, Lunde PK, et al. Intrinsic skeletal muscle alterations in chronic heart failure patients: a disease-specific myopathy or a result of deconditioning? Heart Fail Rev 2012;17:421-36. [PubMed]
- Morley JE. Sarcopenia in the elderly. Fam Pract 2012;29 Suppl 1:i44-i48. [PubMed]
- Budui SL, Rossi AP, Zamboni M. The pathogenetic bases of sarcopenia. Clin Cases Miner Bone Metab 2015;12:22-6. [PubMed]
- Crescenzo R, Bianco F, Mazzoli A, et al. Skeletal muscle mitochondrial energetic efficiency and aging. Int J Mol Sci 2015;16:10674-85. [PubMed]
- Denison HJ, Cooper C, Sayer AA, et al. Prevention and optimal management of sarcopenia: a review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin Interv Aging 2015;10:859-69. [PubMed]
- Menon MK, Houchen L, Singh SJ, et al. Inflammatory and satellite cells in the quadriceps of patients with COPD and response to resistance training. Chest 2012;142:1134-42. [PubMed]
- Barreiro E, Ferrer D, Sanchez F, et al. Inflammatory cells and apoptosis in respiratory and limb muscles of patients with COPD. J Appl Physiol (1985) 2011;111:808-17. [PubMed]
- Crul T, Spruit MA, Gayan-Ramirez G, et al. Markers of inflammation and disuse in vastus lateralis of chronic obstructive pulmonary disease patients. Eur J Clin Invest 2007;37:897-904. [PubMed]
- Fermoselle C, Rabinovich R, Ausín P, et al. Does oxidative stress modulate limb muscle atrophy in severe COPD patients? Eur Respir J 2012;40:851-62. [PubMed]
- Barreiro E. Protein carbonylation and muscle function in COPD and other conditions. Mass Spectrom Rev 2014;33:219-36. [PubMed]
- Gouzi F, Abdellaoui A, Molinari N, et al. Fiber atrophy, oxidative stress, and oxidative fiber reduction are the attributes of different phenotypes in chronic obstructive pulmonary disease patients. J Appl Physiol (1985) 2013;115:1796-805. [PubMed]
- Marin-Corral J, Minguella J, Ramírez-Sarmiento AL, et al. Oxidised proteins and superoxide anion production in the diaphragm of severe COPD patients. Eur Respir J 2009;33:1309-19. [PubMed]
- Rabinovich RA, Ardite E, Mayer AM, et al. Training depletes muscle glutathione in patients with chronic obstructive pulmonary disease and low body mass index. Respiration 2006;73:757-61. [PubMed]
- Wijnhoven HJ, Heunks LM, Geraedts MC, et al. Oxidative and nitrosative stress in the diaphragm of patients with COPD. Int J Chron Obstruct Pulmon Dis 2006;1:173-9. [PubMed]
- Wijnhoven JH, Janssen AJ, van Kuppevelt TH, et al. Metabolic capacity of the diaphragm in patients with COPD. Respir Med 2006;100:1064-71. [PubMed]
- Orozco-Levi M, Coronell C, Ramírez-Sarmiento A, et al. Injury of peripheral muscles in smokers with chronic obstructive pulmonary disease. Ultrastruct Pathol 2012;36:228-38. [PubMed]
- Camillo CA, Burtin C, Hornikx M, et al. Physiological responses during downhill walking: A new exercise modality for subjects with chronic obstructive pulmonary disease? Chron Respir Dis 2015;12:155-64. [PubMed]
- Martínez-Llorens J, Casadevall C, Lloreta J, et al. Activation of satellite cells in the intercostal muscles of patients with chronic obstructive pulmonary disease. Arch Bronconeumol 2008;44:239-44. [PubMed]
- Thériault ME, Paré MÈ, Lemire BB, et al. Regenerative defect in vastus lateralis muscle of patients with chronic obstructive pulmonary disease. Respir Res 2014;15:35. [PubMed]
- Pomiès P, Rodriguez J, Blaquière M, et al. Reduced myotube diameter, atrophic signalling and elevated oxidative stress in cultured satellite cells from COPD patients. J Cell Mol Med 2015;19:175-86. [PubMed]
- Thériault ME, Paré MÈ, Maltais F, et al. Satellite cells senescence in limb muscle of severe patients with COPD. PLoS One 2012;7:e39124. [PubMed]
- Agustí AG, Sauleda J, Miralles C, et al. Skeletal muscle apoptosis and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:485-9. [PubMed]
- Guo Y, Gosker HR, Schols AM, et al. Autophagy in locomotor muscles of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;188:1313-20. [PubMed]
- Puig-Vilanova E, Ausin P, Martinez-Llorens J, et al. Do epigenetic events take place in the vastus lateralis of patients with mild chronic obstructive pulmonary disease? PLoS One 2014;9:e102296. [PubMed]
- Puig-Vilanova E, Martínez-Llorens J, Ausin P, et al. Quadriceps muscle weakness and atrophy are associated with a differential epigenetic profile in advanced COPD. Clin Sci (Lond) 2015;128:905-21. [PubMed]
- Jatta K, Eliason G, Portela-Gomes GM, et al. Overexpression of von Hippel-Lindau protein in skeletal muscles of patients with chronic obstructive pulmonary disease. J Clin Pathol 2009;62:70-6. [PubMed]
- Jobin J, Maltais F, Doyon JF, et al. Chronic obstructive pulmonary disease: capillarity and fiber-type characteristics of skeletal muscle. J Cardiopulm Rehabil 1998;18:432-7. [PubMed]
- Saey D, Michaud A, Couillard A, et al. Contractile fatigue, muscle morphometry, and blood lactate in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;171:1109-15. [PubMed]
- Vogiatzis I, Terzis G, Stratakos G, et al. Effect of pulmonary rehabilitation on peripheral muscle fiber remodeling in patients with COPD in GOLD stages II to IV. Chest 2011;140:744-52. [PubMed]
- Green HJ, Burnett ME, D’Arsigny C, et al. Muscle fiber type characteristics in females with chronic obstructive pulmonary disease. A preliminary study. J Mol Histol 2009;40:41-51. [PubMed]
- Jiménez-Fuentes MA, Gea J, Aguar MC, et al. Capillary density and respiratory function in the external intercostal muscle. Arch Bronconeumol 1999;35:471-6. [PubMed]
- Orozco-Levi M, Gea J, Aguar MC, et al. Changes in the capillary content in the diaphragm of COPD patients: a sort of muscle remodelling? Am J Respir Crit Care Med 1996;153:A298.
- Alexopoulou C, Mitrouska I, Arvanitis D, et al. Vascular-specific growth factor mRNA levels in the human diaphragm. Respiration 2005;72:636-41. [PubMed]
- Poole DC, Mathieu-Costello O. Effect of pulmonary emphysema on diaphragm capillary geometry. J Appl Physiol (1985) 1997;82:599-606. [PubMed]
- de Theije CC, Langen RC, Lamers WH, et al. Differential sensitivity of oxidative and glycolytic muscles to hypoxia-induced muscle atrophy. J Appl Physiol (1985) 2015;118:200-11. [PubMed]
- Gosker HR, Hesselink MK, Duimel H, et al. Reduced mitochondrial density in the vastus lateralis muscle of patients with COPD. Eur Respir J 2007;30:73-9. [PubMed]
- Meyer A, Zoll J, Charles AL, et al. Skeletal muscle mitochondrial dysfunction during chronic obstructive pulmonary disease: central actor and therapeutic target. Exp Physiol 2013;98:1063-78. [PubMed]
- Levine S, Gregory C, Nguyen T, et al. Bioenergetic adaptation of individual human diaphragmatic myofibers to severe COPD. J Appl Physiol (1985) 2002;92:1205-13. [PubMed]
- Ribera F, N’Guessan B, Zoll J, et al. Mitochondrial electron transport chain function is enhanced in inspiratory muscles of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:873-9. [PubMed]
- Sanchez J, Brunet A, Medrano G, et al. Metabolic enzymatic activities in the intercostal and serratus muscles and in the latissimus dorsi of middle-aged normal men and patients with moderate obstructive pulmonary disease. Eur Respir J 1988;1:376-83. [PubMed]
- Sánchez J, Bastien C, Medrano G, et al. Metabolic enzymatic activities in the diaphragm of normal men and patients with moderate chronic obstructive pulmonary disease. Bull Eur Physiopathol Respir 1984;20:535-40. [PubMed]
- Maltais F, LeBlanc P, Whittom F, et al. Oxidative enzyme activities of the vastus lateralis muscle and the functional status in patients with COPD. Thorax 2000;55:848-53. [PubMed]
- Saey D, Lemire BB, Gagnon P, et al. Quadriceps metabolism during constant workrate cycling exercise in chronic obstructive pulmonary disease. J Appl Physiol (1985) 2011;110:116-24. [PubMed]
- Sauleda J, García-Palmer F, Wiesner RJ, et al. Cytochrome oxidase activity and mitochondrial gene expression in skeletal muscle of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1413-7. [PubMed]
- Crul T, Testelmans D, Spruit MA, et al. Gene expression profiling in vastus lateralis muscle during an acute exacerbation of COPD. Cell Physiol Biochem 2010;25:491-500. [PubMed]
- Gosker HR, Zeegers MP, Wouters EF, et al. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax 2007;62:944-9. [PubMed]
- Doucet M, Debigaré R, Joanisse DR, et al. Adaptation of the diaphragm and the vastus lateralis in mild-to-moderate COPD. Eur Respir J 2004;24:971-9. [PubMed]
- Maltais F, Sullivan MJ, LeBlanc P, et al. Altered expression of myosin heavy chain in the vastus lateralis muscle in patients with COPD. Eur Respir J 1999;13:850-4. [PubMed]
- Patel MS, Natanek SA, Stratakos G, et al. Vastus lateralis fiber shift is an independent predictor of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014;190:350-2. [PubMed]
- Gosker HR, Engelen MP, van Mameren H, et al. Muscle fiber type IIX atrophy is involved in the loss of fat-free mass in chronic obstructive pulmonary disease. Am J Clin Nutr 2002;76:113-9. [PubMed]
- Orozco-Levi M, Gea J, Sauleda J, et al. Structure of the latissimus dorsi muscle and respiratory function. J Appl Physiol (1985) 1995;78:1132-9. [PubMed]
- Testelmans D, Crul T, Maes K, et al. Atrophy and hypertrophy signalling in the diaphragm of patients with COPD. Eur Respir J 2010;35:549-56. [PubMed]
- Stubbings AK, Moore AJ, Dusmet M, et al. Physiological properties of human diaphragm muscle fibres and the effect of chronic obstructive pulmonary disease. J Physiol 2008;586:2637-50. [PubMed]
- Engelen MP, Orozco-Levi M, Deutz NE, et al. Glutathione and glutamate levels in the diaphragm of patients with chronic obstructive pulmonary disease. Eur Respir J 2004;23:545-51. [PubMed]
- Brocca L, Pellegrino MA, Lameri E, et al. Contractile properties of single muscle fibres from human intercostal muscles. J Mus Res Cell Motil 2003;24:335.
- Ottenheijm CA, Heunks LM, Sieck GC, et al. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;172:200-5. [PubMed]
- Debigaré R, Côte CH, Hould FS, et al. In vitro and in vivo contractile properties of the vastus lateralis muscle in males with COPD. Eur Respir J 2003;21:273-8. [PubMed]
- Morrison WL, Gibson JN, Scrimgeour C, et al. Muscle wasting in emphysema. Clin Sci (Lond) 1988;75:415-20. [PubMed]
- Engelen MP, Deutz NE, Wouters EF, et al. Enhanced levels of whole-body protein turnover in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:1488-92. [PubMed]
- Pouw EM, Schols AM, Deutz NE, et al. Plasma and muscle amino acid levels in relation to resting energy expenditure and inflammation in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158:797-801. [PubMed]
- Yoneda T, Yoshikawa M, Fu A, et al. Plasma levels of amino acids and hypermetabolism in patients with chronic obstructive pulmonary disease. Nutrition 2001;17:95-9. [PubMed]
- Engelen MP, Wouters EF, Deutz NE, et al. Factors contributing to alterations in skeletal muscle and plasma amino acid profiles in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 2000;72:1480-7. [PubMed]
- Jagoe RT, Engelen MP. Muscle wasting and changes in muscle protein metabolism in chronic obstructive pulmonary disease. Eur Respir J Suppl 2003;46:52s-63s. [PubMed]
- Costes F, Gosker H, Feasson L, et al. Impaired exercise training-induced muscle fiber hypertrophy and Akt/mTOR pathway activation in hypoxemic patients with COPD. J Appl Physiol (1985) 2015;118:1040-9. [PubMed]
- Debigaré R, Côté CH, Maltais F. Peripheral muscle wasting in chronic obstructive pulmonary disease. Clinical relevance and mechanisms. Am J Respir Crit Care Med 2001;164:1712-7. [PubMed]
- Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004;117:399-412. [PubMed]
- Cai D, Frantz JD, Tawa NE Jr, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 2004;119:285-98. [PubMed]
- Doucet M, Russell AP, Léger B, et al. Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;176:261-9. [PubMed]
- Plant PJ, Brooks D, Faughnan M, et al. Cellular markers of muscle atrophy in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2010;42:461-71. [PubMed]
- Ottenheijm CA, Heunks LM, Li YP, et al. Activation of the ubiquitin-proteasome pathway in the diaphragm in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;174:997-1002. [PubMed]
- Scott SV, Klionsky DJ. Delivery of proteins and organelles to the vacuole from the cytoplasm. Curr Opin Cell Biol 1998;10:523-9. [PubMed]
- Supinski GS, Callahan LA. Caspase activation contributes to endotoxin-induced diaphragm weakness. J Appl Physiol (1985) 2006;100:1770-7. [PubMed]
- Wagner PD. Skeletal muscles in chronic obstructive pulmonary disease: deconditioning, or myopathy? Respirology 2006;11:681-6. [PubMed]