Surgical techniques and outcome analysis of uniportal video-assisted thoracic surgery complex sleeve lung resection: a 20 case-series study
Introduction
Video-assisted thoracic surgery (VATS) was first used for the surgical treatment of non-small cell lung cancer (NSCLC) in the early 1990s. After more than 30 years of development, VATS has demonstrated outcomes comparable to thoracotomy and is now the treatment of choice for early-stage NSCLC (1).
Migliore et al. pioneered uniportal thoracoscopic surgery in 1998 (2). However, the concept of single-port thoracoscopic surgery was first proposed by Rocco et al. in 2004 (3). Subsequently, Rocco et al. reported more than 600 cases of uniportal VATS lung wedge resections and biopsies (4). More recently, Gonzalez and colleagues were the first to report anatomical pulmonary sleeve resections and mediastinal tumor resections via the uniportal technique (5,6).
From June 2016 to April 2020, a total of 183 cases of uniportal VATS sleeve pulmonary resections were performed by the single surgical team at the thoracic surgery department of the Shanghai Pulmonary Hospital. Of these, 163 cases of the upper, middle, and lower lobe sleeve resections have already been analyzed in previous reports (7-12). This article aims to analyze the remaining 20 cases, which we categorize as “complex sleeve lung resections ”. This study aims to explore the feasibility of uniportal VATS complex sleeve lung resections and to summarize the surgical techniques and clinical outcomes.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-3002).
Methods
Clinical data
This retrospective study of 20 complex sleeve pulmonary and pulmonary-sparing bronchial and distal tracheal resections was conducted from June 2016 to April 2020 via the uniportal VATS technique. We included cases requiring (I) pulmonary segment sleeve resection or extended sleeve resection (sleeve lobectomy plus segmentectomy of the remaining lobe); (II) sleeve pulmonary resection (lobectomy and pneumonectomy) involving the carina, and (III) distal tracheal tumor resection. We did not include 163 routine sleeve lobectomy and bilobectomy cases or cases requiring a wedge bronchoplasty. The recruited cases were four pulmonary segment sleeve resections (two tri-segment and two lingula sleeve resections), six left lower lobe plus lingula sleeve resections, and nine carinal sleeve resections (two sleeve pneumonectomies, four right upper lobe sleeve resections, and carina reconstructions, one right main bronchial segmental resection with double carina reconstruction, one left upper lobe sleeve resection and carina reconstruction, one right bilobectomy sleeve resection with carinal reconstruction, and one distal trachea resection).
There were 17 males and 3 females, aged 58.3±11.74 years old. The main clinical manifestations were cough and bloody sputum, while five patients had no special symptoms, and their diseases were found during physical examination. There were 11 smokers, 15 patients with mild-moderate pulmonary function compromise, and eight patients with cardiopulmonary or other comorbidities (such as high blood pressure or diabetes). One patient received preoperative chemotherapy. The preoperative examinations included a computed tomography (CT) scan of the chest, positron emission tomography scan, bone scan, and bronchoscopy. Brain magnetic resonance imaging (MRI) was performed in all patients. Chest CT scans were visualized by 3D-reconstruction of the pulmonary vessels and the bronchial tree and were used for preoperative planning. Bronchoscopy was used to obtain tissue diagnosis and for preoperative planning. Pulmonary function tests (PFTs), electrocardiograms, and echocardiography were used for the patients’ routine physiologic evaluation (Table 1).
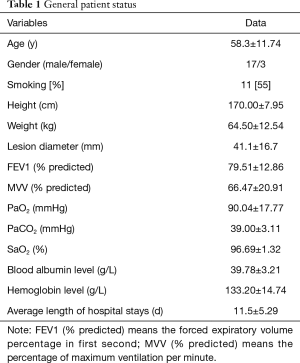
Full table
Perioperative treatment
Preoperative routine sputum cultures, lung function, and electrocardiograms were performed for all patients . Patients whose blood count showed elevated white blood cells and who had a poor pulmonary function, especially those with a history of chronic obstructive pulmonary disease, were given antibiotics. Patients were encouraged to quit smoking at least one week before the operation. Mucolytic drugs and bronchodilators were routinely administered, and respiratory physiotherapy was applied. A chest X-ray was obtained on a postoperative day (POD) 1. Active sputum suction was performed if patients had difficulty managing their secretions. If the operation included a vascular anastomosis, preventive anticoagulation treatment with low molecular weight heparin (LMWH) was given while in hospital and then changed to aspirin for three months post-discharge. Chest drains were removed in the absence of air leakage and if drainage was less than 250 mL in 24 hours.
Surgical technique
The patients were intubated with a double-lumen endotracheal tube under general anesthesia and placed in the lateral decubitus position. A 4 cm incision was made in the 4th intercostal space between the middle and posterior axillary line. The treatment methods for blood vessels, pulmonary fissures, and lymph nodes were the same as for complete thoracoscopic lobectomy. The specific steps were as follows: sleeve bronchial resection, anastomosis trimming, and frozen pathological examination to ensure that there was no tumor infiltration at the incision margin; then 3-0 (tracheal and leaf bronchial anastomosis) or 4-0 (segmental bronchial anastomosis) non-absorbable sutures (prolene, Covidien Inc., USA) were sutured continuously in clockwise and counterclockwise directions from the medial wall, respectively, sutured from the back of the anastomotic site to the front, knotted after suturing, and tested through flushing for no leakage even when the airway pressure was maintained at 30 cmH2O.
Uniportal VATS special sleeve lung resection needs to follow the conventional surgical technique of uniportal VATS sleeve lung resection, which has been summarized in previous articles (7-12).
Uniportal VATS complex sleeve pulmonary resections
- Left lower lobe + lingula sleeve resection: the intruded upper lobe lingular segment of the lower lobe of the left lung was excised, and the bronchus of the propria segment of the upper lobe was anastomosed with the left main bronchus end to end.
- Left upper lobe propria segment sleeve resection: the propria segment of the upper lobe of the left lung was excised, and the bronchus of the lingula was anastomosed to the bronchus of the upper lobe (Figure 1).
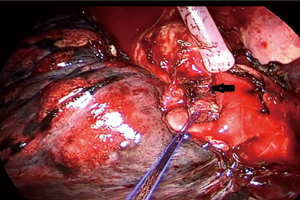 Figure 1 VATS sleeve resection of the left upper lobe inherent segment: the left upper lobe inherent segment is removed, and then the bronchus of the lingual segment of the upper lobe is anastomosed end to end with the bronchus of the upper lobe. (← is the bronchus of the upper lobe). VATS, video-assisted thoracic surgery.
Figure 1 VATS sleeve resection of the left upper lobe inherent segment: the left upper lobe inherent segment is removed, and then the bronchus of the lingual segment of the upper lobe is anastomosed end to end with the bronchus of the upper lobe. (← is the bronchus of the upper lobe). VATS, video-assisted thoracic surgery. - Lingula sleeve resection: the lingular was excised, and the bronchus of the propria segment of the upper lobe was anastomosed with the bronchus of the upper lobe end to end.
- Right upper lobe + carinoplasty resection: the upper lobe of the right lung and the right half carina were excised, and the bronchus intermedius was anastomosed to the right lateral wall of the lower trachea.
- Right upper lobe + carina resection: the left main bronchus was first anastomosed with the trachea by two-thirds of the pipe diameter, and the bronchus intermedius was then anastomosed to the gap remaining after the left main bronchus was anastomosed with the trachea (Figure 2).
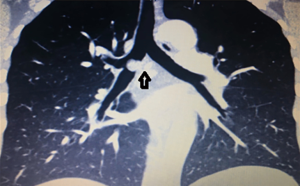
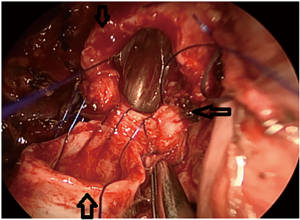 Figure 2 VATS right upper lobe and carina resection: remove the right upper lobes and remove all the carinas. The left main bronchus is anastomosed with the trache at the first two-thirds of the pipe diameter, and the right middle bronchus is anastomosed to the left main bronchus and trachea after anastomosis. ↑ (↓ is the trachea, ←is the left main bronchus, ↑ is the right middle bronchus). VATS, video-assisted thoracic surgery.
Figure 2 VATS right upper lobe and carina resection: remove the right upper lobes and remove all the carinas. The left main bronchus is anastomosed with the trache at the first two-thirds of the pipe diameter, and the right middle bronchus is anastomosed to the left main bronchus and trachea after anastomosis. ↑ (↓ is the trachea, ←is the left main bronchus, ↑ is the right middle bronchus). VATS, video-assisted thoracic surgery. - Right main bronchus and second carina resection with double carinoplasty: the bronchus intermedius and the right upper lobe bronchus were completely cut off 1 cm from the lower edge of the tumor, and the right main bronchus and the outer wall of the lower trachea were wedged 1 cm from the upper edge of the tumor. The bronchus intermedius was anastomosed with the right upper bronchus’s medial wall to form a new secondary carina, which was anastomosed to the trachea at the level of the orifice of the resected right main bronchus (Figures 3,4
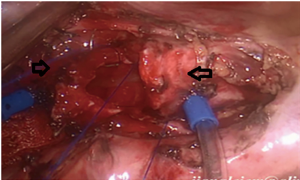
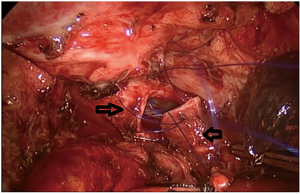
). 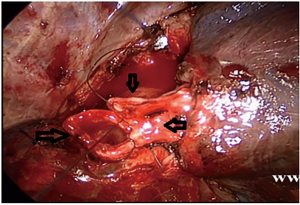 Figure 4 Uniportal VATS intercostal, right main bronchial segment resection, and double carina angioplasty: the right middle bronchus and the right upper bronchus are completely severed 1 cm from the lower margin of the tumor, and the lateral walls of the right main bronchus and the lower trachea are cut in a wedge shape 1 cm from the upper margin of the tumor. The second carina is reconstructed by continuous side to side anastomosis of the right middle bronchus with the residual branch of the medial wall of the right upper lobe bronchus, and the second carina is anastomosed end to end with the lower end of the trachea to reconstruct a new carina. (→ is the right middle bronchus, ← is the right upper bronchus, ↓ is the trachea). VATS, video-assisted thoracic surgery.
Figure 4 Uniportal VATS intercostal, right main bronchial segment resection, and double carina angioplasty: the right middle bronchus and the right upper bronchus are completely severed 1 cm from the lower margin of the tumor, and the lateral walls of the right main bronchus and the lower trachea are cut in a wedge shape 1 cm from the upper margin of the tumor. The second carina is reconstructed by continuous side to side anastomosis of the right middle bronchus with the residual branch of the medial wall of the right upper lobe bronchus, and the second carina is anastomosed end to end with the lower end of the trachea to reconstruct a new carina. (→ is the right middle bronchus, ← is the right upper bronchus, ↓ is the trachea). VATS, video-assisted thoracic surgery. - Right sleeve (carina) pneumonectomy (Figure 5).
- Left upper lobe + carinoplasty resection: the left upper lobe and the left half of the carina were excised, and the left lower lobe bronchus was sutured to the lateral wall of the lower segment of the trachea (Figure 6).
- Tracheal tumor resection and an end to end anastomosis: the tracheal tumor was excised, and the trachea was anastomosed end to end.
Challenges during uniportal VATS complex sleeve pulmonary resections
Exposure of the proximal and distal bronchus during the anastomosis
The exposure of the anastomosis was blocked by the pulmonary artery, which laid anteriorly to the bronchus. This mainly occurred during the lingula bronchus’s anastomosis to the left upper lobe bronchus’s orifice following a tri-segment sleeve resection. To improve exposure and construct the anastomosis, we used tourniquets to ligate and retract the pulmonary artery temporarily. By temporarily ligating the artery, its diameter was reduced significantly, improving the proximal and distal stumps’ exposure. By suspending the pulmonary artery above the anastomosis level and retracting it posteriorly, the bronchial stumps were completely exposed, facilitating the anastomosis (Figure 1).
Caliber discrepancy between the proximal and distal bronchial stumps
A discrepancy in the caliber between the proximal and distal bronchial stumps is a challenge encountered mostly when anastomosing the segmental bronchus to the lobar bronchus following a segmental sleeve resection. If it is not addressed properly, it will become a problem that may lead to anastomotic-related complications such as stenosis or even dehiscence. In our series, such a discrepancy occurred in the cases of propria sleeve resections, after which the lingula bronchus was anastomosed to the orifice of the left upper bronchus. We obliquely trimmed the smaller bronchus to tackle the discrepancy in the caliber of the bronchial stumps. This increased the length of the perimeter of the lingula bronchus. Also, we adjusted the pace of the suturing accordingly. The pace was smaller on the lingula bronchus and larger on the orifice of the left upper bronchus (Figure 1).
Deep tissue exposure
This mainly occurred in sleeve resections involving the carina, where the anastomosis was difficult due to the deeply situated carina or main bronchus. When this occurred, the deeply situated trachea that needed anastomosis could be made more shallow by pulling the trachea nearby to improve the surrounding tissues’ exposure. The pull suture could be sutured to the posterior chest wall or drawn out of the chest wall by a deep venipuncture cannula.
Airway management
This challenge mainly occurred in the carina’s operations, such as uniportal VATS sleeve (carina) right pneumonectomies. In these cases, we used a specially modified double-lumen endotracheal tube to ventilate the left main bronchus . During the anastomosis of the left main bronchus with the trachea, high-frequency jet ventilation (HFJV) was applied by a catheter that had been previously inserted into the left main bronchus via the bronchial lumen of the modified endotracheal tube, which was withdrawn above the level of the carina into the distal trachea. When the posterior aspect of the anastomosis was completed, the endotracheal tube was repositioned in the initial position, HFJV was discontinued, the catheter was withdrawn, and ventilation via the endotracheal tube resumed (Figure 5). According to our clinical experience, in general, an adult female of normal size can be treated with a 35-fr double-lumen endotracheal tube. We used a 32-fr double-lumen endotracheal tube and used 3M stickers to reduce the endotracheal intubation’s balloon size not to contact the tracheal tumor would not increase the anastomosis tension between the left main bronchus and the trachea. A sputum aspiration catheter could be easily inserted into the left main bronchus to prevent the aspiration of blood, sputum, and tumor debris into the left bronchial tree.
Collapse of the bronchial lumen after the anastomosis
This mainly occurred after a tri-segment sleeve resection. After the resection, the lingula bronchus had to be reconnected to the left upper bronchus. Because the lingula bronchus is smaller in caliber and longer than the left upper lobe bronchus, the lingula bronchus stump’s rim can be overstretched during the anastomosis. This can lead to narrowing the lumen of the lingula bronchus, resulting in collapse and obstruction. The distal stump’s perimeter (the lingula bronchus in our case) should be trimmed obliquely and not left too long to avoid this complication. Stitches should be placed close to the rim to avoid the distal bronchial stump’s intussusception into the proximal bronchial stump (the left upper lobe bronchus).
Statistical analysis
Data were collected retrospectively and analyzed using STATA/MP (StataCorp, College Station, TX, USA) and GraphPad Prism (GraphPad Software, San Diego, CA, USA) statistical software. Continuous variables are given as the mean ± standard deviation. Categorical data are presented as frequencies and percentages. The median follow-up time was estimated using the reverse Kaplan-Meier method. Survival rates were calculated, and survival curves were plotted using the Kaplan-Meier method. Univariate analysis was performed by χ2 using SPSS 15.0 (SPSS Inc., USA). A probability level of <0.05 was considered statistically significant.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Shanghai Pulmonary Hospital (NO.: NCT03523468) and informed consent was taken from all the patients.
Results
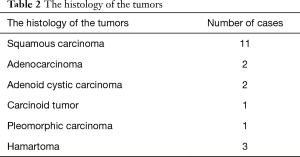
The majority of cases were squamous cell carcinoma of the lung, followed by adenocarcinoma. The histology of the tumors is shown in Table 2.
Full table
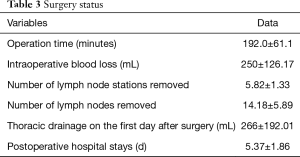
All resections were R0. Twelve cases were found to have the early-stage disease (stages I–II), while seven cases had the advanced-stage disease (stage III) locally. Table 2 shows the patients’ stage distributions. The average operation time was 192.0±61.1 minutes. A median number of 5.82±1.33 lymph node stations was sampled. Subcarinal lymph nodes were always sampled. The median number of lymph nodes sampled was 4.18±5.89. The average intraoperative blood loss was 250±126.17 mL (50–800 mL). Two patients were transfused with blood intraoperatively. The average drainage output was 266±192.01 mL. The average length of stay (LOS) after the operation was 5.37±1.86 days. No case in this group was ever converted into tri-portal VATS or open surgery (Table 3).
Full table
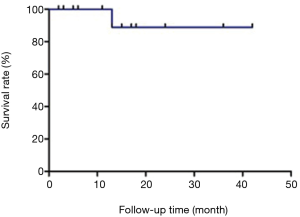
Among the malignant tumor cases, 12 patients received postoperative chemotherapy (12/16). The 30-day mortality rate was zero (Figure 7). Postoperative anastomotic-related complications included one stenosis (1/20). In this case of a left upper lobe sleeve, anastomotic stenosis was found 14 months after the operation due to granuloma formation. The stenosis was resolved following repetitive sessions of cauterization and dilatation. A local recurrence in a patient with a squamous cell carcinoma was treated by a right lower bilobectomy. In this case, the right main bronchus was also resected, and the remaining upper lobe bronchus was anastomosed to the carina. Anastomotic stenosis was found eight months after the operation, with pathology suggesting local recurrence. A stent was inserted, and systemic therapy was initiated. Another patient died of a bronchial esophageal fistula one year after the operation. This patient had undergone a left lower lobe and lingula sleeve resection due to a lower lobe’s squamous cell carcinoma that also extended to the lingula and had received postoperative chemoradiotherapy. At the time of his death, he already had extensive distant metastasis.
Discussion
Bronchial sleeve lobectomy was initially adopted to treat central lung cancer, with the first sleeve resection (right upper lobe) performed by Dr. Allison in 1952. In 2002, Santambrogio et al. (13) reported the first complete thoracoscopic sleeve lobectomy to treat the left lower lobe’s mucoepidermoid carcinoma. Compared with conventional VATS, the uniportal technique is less invasive, as it utilizes a single 3–4 cm incision, unlike multiple ports of other VATS techniques. Although it can be performed with conventional instruments used for open surgery, it is more convenient to use specially designed long (ideally more than 30 cm), thin-shafted (5–7 mm), double-articulated instruments. Currently, bronchial sleeve lobectomy accounts for 5–13% of total lung cancer resections (14). The incidence of postoperative complications and mortality has been reported as approximately 11.6% and 2–6%, respectively (14). The advantage of a sleeve lobectomy includes preserving healthy lung tissue, which is associated with fewer postoperative complications and a better quality of life post-surgery—especially the possibility of intratubular malignant lesions or low-grade malignant lesions considered for surgery. However, there is no clear basis for malignancy. We can consider sleeve segment resection of the lung. We had three hamartoma cases in our group. Therefore, it has been widely adopted in clinical practice.
Currently, uniportal VATS sleeve resection mainly includes right upper lobe sleeve resection, left upper lobe sleeve resection, and left lower lobe sleeve resection. To date, there have been few reports of uniportal VATS complex sleeve lung resections. This may be due to the extensive prior experience required to complete a complex sleeve procedure via the uniportal VATS technique. Gonzalez-Rivas et al. have suggested that 200 uniportal VATS standard lobectomies and 20 open sleeve lobectomies should be conducted before attempting a uniportal VATS sleeve lobectomy. As such, the experience required to undertake complex uniportal VATS sleeve lobectomies is even greater (6). Uniportal VATS complex sleeve lung resections need to follow the conventional surgical techniques for uniportal VATS sleeve lung resection, which have been summarized in previous articles (7-12). However, special techniques should be applied according to the characteristics of the complex sleeve surgery.
The technical principles of a uniportal VATS complex resection, such as those described above, are generally not different from those applied for a standard uniportal VATS lobectomy. However, certain challenges are not present when dealing with a standard resection, whether uniportal or multiportal. As defined earlier, a complex resection involves the division and reconstruction of the bronchial tree, which is not required during a standard procedure. It is crucial to construct a flawless anastomosis to avoid anastomotic-related complications in the postoperative period, such as stenosis, dehiscence, or bronchopleural fistula. This means that the proximal and distal bronchial stumps should be adequately exposed and reconnected evenly with precise sutures. In the left side, especially during a left upper bronchial sleeve procedure, the bronchus is situated posterior to the pulmonary artery. Because uniportal VATS is mainly an anterior-to-posterior procedure, this means that the artery may obscure our vision of the bronchial stumps and limit the space required for suturing. By temporarily ligating the pulmonary artery with tourniquets and suspending it, we can improve our vision of the stumps and gain the space required to suture them together.
Another challenge is that of the caliber discrepancy between the distal and proximal stumps. This can lead to failure of the anastomosis resulting in stenosis or dehiscence. The discrepancy between the distal and proximal stumps is most evident when attempting to anastomose a lobar bronchus to the carina or a segmental bronchus to the main bronchus. Trimming the stumps obliquely and adjusting the suture’s pace (larger in the distal stump, smaller in the proximal stump) is an efficient way to deal with this challenge.
While airway management is not a problem during a standard uniportal VATS lobectomy or even a sleeve lobectomy, it is crucial when a resection of the carina is required. Measures to control the airway and achieve sufficient ventilation include a cardiopulmonary bypass (CPB), extracorporeal membrane oxygenation (ECMO), and HFJV (15). CPB and ECMO are more invasive than HFJV and, in most cases, are not necessary. The use of HFJV significantly facilitates the carina’s reconstruction when sutures are being placed in the posterior (membranous) part of the anastomosis, where the presence of an endobronchial tube would obscure the vision and the exposure of the stump rims.
The incidence of bronchopleural fistula and anastomotic stenosis was reported as 0–6% and 3–9%, respectively, in patients undergoing sleeve lobectomy through thoracotomy (16). Our research group recently published the outcomes of 79 cases of uniportal VATS sleeve lobectomies conducted over almost 4 years. The incidence of anastomotic-related complications in this series was zero (12). In the present study, the incidence of anastomotic fistula and stenosis was 4.3%, consistent with other literature (17). Extensive dissection of the bronchi, carina, or trachea beyond the anastomosis level should be avoided to avoid the occurrence of anastomotic complications. Tension and torsion of the anastomosis should also be avoided. In the longer term, the risk of anastomotic leakage may arise due to postoperative radiotherapy.
Okada et al. reported a local recurrence rate of 8% after routine bronchus sleeve lobectomy (18), and Gezer et al. reported a local recurrence rate of 11.7% (19). In this study, the local recurrence incidence was 5%, which was similar to that after conventional bronchial sleeve lobectomy. One patient in this group had a large tumor with a diameter greater than 7 cm, which extended across the pulmonary fissure involving the other lobe. The mediastinal lymph nodes had also been infiltrated. Due to the patient’s poor pulmonary function, a pneumonectomy would not have been well tolerated. Local recurrence is related to the pathological stage, the degree of lymph node dissection, and the resection margin status. Especially regarding the resection margins, intraoperative confirmation of disease-free margins should be obtained via frozen section pathologic examination. If the margins are found to be positive, a pneumonectomy instead of a sleeve lobectomy should be performed. Neoadjuvant chemotherapy or immunotherapy and radiotherapy may contribute to R0 resections and should be applied when there is doubt that an R0 resection is feasible (20). However, some surgeons argue that preoperative chemoradiation may increase the rate of postoperative complications (21).
In terms of survival, Wang et al. reported that the one-, three-, and five-year survival rates of patients undergoing bronchial sleeve lobectomy for lung cancer were 81.8%, 52.6%, and 42.3%, respectively (17). Our group’s postoperative follow-up time was 15.6±10.7 months (minimum 3 months, maximum 42 months). In our series, one-year overall survival (OS) was 96.1%. We will be able to provide longer-term OS rates in the future.
The main limitations of our study are its retrospective nature and the limited follow-up period. Also, because the study reflects a single center’s experience and the sample size is not statistically robust, it is not safe to extrapolate the favorable outcomes that we have reported. However, our study does provide favorable outcomes and suggests that uniportal VATS complex pulmonary resections are safe and feasible in experts’ hands.
Acknowledgments
We thank Prof. Yuming Zhu, Chang Chen, Gening Jiang, D. Fitzgerald and J. Chapnick for all their help in preparing this work.
Funding: This study was supported by a grant from the Shanghai Pulmonary Hospital (0823). We had full control of the study design, methods used, outcome measurements, data analysis, and production of the written report.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-3002
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-3002
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-3002
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3002). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Shanghai Pulmonary Hospital (NO.: NCT03523468) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhong Y, Wang Y, Hu X, et al. A systematic review and meta-analysis of thoracoscopic versus thoracotomy sleeve lobectomy. J Thorac Dis 2020;12:5678-90. [Crossref] [PubMed]
- Migliore M, Deodato G. A single-trocar technique for minimally-invasive surgery of the chest. Surg Endosc 2001;15:899-901. [Crossref] [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Rocco G, Martucci N, La Manna C, et al. Ten-year experience on 644 patients undergoing single-port (uniportal) video-assisted thoracoscopic surgery. Ann Thorac Surg 2013;96:434-8. [Crossref] [PubMed]
- Gonzalez D, de la Torre M, Paradela M, et al. Video-assisted thoracic surgery lobectomy: 3-year initial experience with 200 cases. Eur J Cardiothorac Surg 2011;40:e21-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections†. Eur J Cardiothorac Surg 2016;49:i6-16. [PubMed]
- Wu L, Wang H, Cai H, et al. Comparison of Double Sleeve Lobectomy by Uniportal Video-Assisted Thoracic Surgery (VATS) and Thoracotomy for NSCLC Treatment. Cancer Manag Res 2019;11:10167-74. [Crossref] [PubMed]
- Venkitaraman B, Lei J, Liang W, et al. Uniportal video-assisted thoracoscopy surgery in lung cancer: largest experience. Asian Cardiovasc Thorac Ann 2019;27:559-64. [Crossref] [PubMed]
- Qu J, Zhu Y, Zhao D, et al. Clinical analysis of 114 cases of uniportal thoracoscopy sleeve lobectomy. Zhonghua Wai Ke Za Zhi 2018;56:938-40.
- Abu Akar F, Yang C, Lin L, et al. Intra-pericardial double sleeve uniportal video-assisted thoracoscopic surgery left upper lobectomy. J Vis Surg 2017;3:51. [Crossref] [PubMed]
- Yang C, Abu Akar F, Chen J, et al. Right sleeve pneumonectomy via uniportal video-assisted thoracoscopic approach. J Thorac Dis 2018;10:E391-6. [Crossref] [PubMed]
- Soultanis KM, Chen Chao M, Chen J, et al. Technique and outcomes of 79 consecutive uniportal video-assisted sleeve lobectomies. Eur J Cardiothorac Surg 2019;56:876-82. [Crossref] [PubMed]
- Santambrogio L, Cioffi U, De Simone M, et al. Video-assisted sleeve lobectomy for mucoepidermoid carcinoma of the left lower lobar bronchus: a case report. Chest 2002;121:635-6. [Crossref] [PubMed]
- Ibrahim M, Venuta F, Rendina EA. Bronchial and pulmonary arterial sleeve resection. Multimed Man Cardiothorac Surg 2005;2005:mmcts 2004 000067.
- Weder W, Inci I. Carinal resection and sleeve pneumonectomy. J Thorac Dis 2016;8:S882-8. [Crossref] [PubMed]
- Martin-Ucar AE, Chaudhuri N, Edwards JG, et al. Can pneumonectomy for non-small cell lung cancer be avoided? An audit of parenchymal sparing lung surgery. Eur J Cardiothorac Surg 2002;21:601-5. [Crossref] [PubMed]
- Wang C, Zhang Z, Gong L, et al. Comparative analysis of sleeve resection and pneumonectomy for lung cancer. Zhongguo Fei Ai Za Zhi 2006;9:18-21. [PubMed]
- Okada M, Yamagishi H, Satake S, et al. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. J Thorac Cardiovasc Surg 2000;119:814-9. [Crossref] [PubMed]
- Gezer S, Oz G, Findik G, et al. Sleeve resections for squamouscell carcinoma of the lung. Heart Lung Circ 2010;19:549. [Crossref] [PubMed]
- Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786-95. [Crossref] [PubMed]
- Krantz SB, Mitzman B, Lutfi W, et al. Neoadjuvant Chemoradiation Shows No Survival Advantage to Chemotherapy Alone in Stage IIIA Patients. Ann Thorac Surg 2018;105:1008-16. [Crossref] [PubMed]