Correlation between epidermal growth factor receptor mutations and nuclear expression of female hormone receptors in non-small cell lung cancer: a meta-analysis
Introduction
Lung cancer, predominantly non-small cell lung cancer (NSCLC), is the leading cause of cancer-related mortality in males and the second cause of cancer-related mortality in females (1). Despite smoking is the predominant risk factor for lung cancer, tobacco can explain only 75% of its incidence (2). A large proportion of lung cancer patients are never-smokers and this phenomenon is more frequent occurred in females and the adenocarcinoma cell type (3).
Extensive reports confirmed that estrogen not only impacts normal lung cell differentiation and maturation, but also affect the growth of lung cancer. It has been reported that estrogen receptor α (ER-α) expressed in the nucleus (0-45%) and cytoplasm (0-73%) of malignant lung cancer cells (4-7). And estrogen receptor β (ER-β) seems to be more coincident that it express only in nuclear in 46% to 60% of NSCLC cases (4-9). Estrogen not only stimulates the transcription of estrogen-responsive genes of lung cells directly but also transactivates the epidermal growth factor receptor (EGFR) pathway (7).
Compared with male, female NSCLC patients respond better when treated with EGFR inhibitors such as gefitinib. EGFR mutation constitutes a larger proportion of lung cancers in female than male, in East Asians than other ethnic and associated with a longer period of fertility (10). EGFR mutation occurs more frequently in female patients and it is the hormone that distinguishes male and female. It seems that a potential connection between female hormone and EGFR mutation. Some studies implied that estrogen interacts with the development of lung cancer. A post hoc analysis suggested that the lung cancer patients may increase the risk of death when treat with hormone replacement therapy (11). Postoperative breast cancer patients treat with tamoxifen will increase the risk of lung cancer (12). In addition, data from the present study showing that EGFR protein expression is down-regulated in response to estrogens and up-regulated in response to antiestrogens (13). In clinic, we observed that significant rash and masculinization occur in patients who had good responds to EGFR-tyrosine kinase inhibitor (TKI). We hypothesize that there is an interaction between estrogen level and EGFR mutated tumors. These studies suggest that ER pathway might be associated with the EGFR pathway.
ER and progesterone receptor (PR) played a pivotal role in estrogen pathway. Previous studies provided controversial results and were limited by small sample size. A more comprehensive analysis was warranted. Therefore, we performed this meta-analysis to synthesize current evidence.
Materials and methods
Literature search
All relevant articles were retrieved by searching PubMed, Embase and the Central Registry of Controlled Trials of the Cochrane Library using a combination of the terms: “ER” or “estrogen receptor” or “PR” or “progesterone receptor” and “lung”. No restriction by language or year was set in the search. The last research time was October 23, 2014.
Data collection
Eligible studies should meet the following criteria: (I) studies which evaluated the association between nuclear ER or PR and EGFR mutation in NSCLC; (II) studies published in English regardless of publication time; (III) the original papers containing enough data. Studies failed to meet the inclusion criteria will be excluded.
Statistical analysis
The results were reported as pooled odds radios (ORs) with the corresponding 95% confidence interval (CI). The pooled OR and its 95% CIs were calculated by the methods proposed by Mantel and Haenszel. Statistical heterogeneity across studies was assessed with a forest plot and the inconsistency statistic (I2). In consideration of any potential heterogeneity, we conducted this meta-analysis with a random-effect model in order to avoid any potential heterogeneity. Subgroup and sensitivity analysis were stratified for predisposed factors when available. Statistical significance was considered at P<0.05. All calculations were performed using Review Manager (version 5.0 for Windows; the Cochrane Collaboration, Oxford, UK)
Publication bias
An extensive search strategy was made to minimize the potential for publication bias. Graphical funnel plots were generated to visually assess a publication bias (14). The statistical methods to detect funnel plot asymmetry were the rank correlation test of Begg and Mazumdar and the regression asymmetry test of Egger (14,15).
Results
Eligible studies
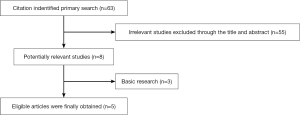
We identified 63 potentially relevant records through the search strategy. Fifty-five studies was excluded after checking the title and abstract, for it was very clear that their research contents didn’t meet our inclusion criteria. Then the full texts of eight articles were carefully screened, three studies were excluded as basic research. At last, a total of five studies were eligible for the final analysis. Figure 1 summarized the flow chart. Among these studies, the relationship between ER-α and EGFR mutation were estimated in five studies, and there are four results for ER-α in Raso’s article (including ER-α3 and ER-α4 respectively expression in nucleus and cytoplasm) only ER-α3 and ER-α4 expression in nucleus were kept in the results. And there are three studies estimated the relationship between ER-β and EGFR. Table 1 summarized the characteristics of all involved studies.

Full table
Relationship between EGFR mutation and expression of ER and PR
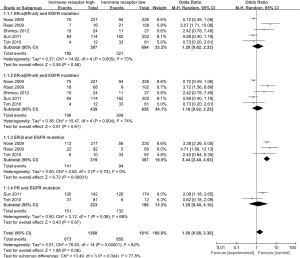
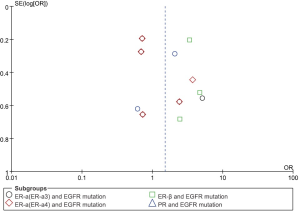
According to all studies with useful data, patients with high ER-β had positive EGFR mutation than low ER-β patients (44.2% vs. 23.7%), and there was a significant difference between the two groups (OR 3.44, 95% CI: 2.40-4.93, test for overall effect: Z=6.72, P<0.001; heterogeneity: χ2=0.62, P=0.73, I2=0%; Figure 2). No significant difference of EGFR mutation can be found between patients with high and low PR expression (OR 1.29, 95% CI: 0.40-4.10, test for overall effect: Z=0.43, P=0.67; heterogeneity: χ2=3.12, P=0.08, I2=68%). Similar results were observed in the analysis of high ER-α and EGFR mutation (here are two results for ER-α in Raso’s article, when included ER-α3, OR 1.20, 95% CI: 0.62-2.33, test for overall effect: Z=0.55, P=0.58; heterogeneity: χ2=14.92, P=0.005, I2=73%; and when included ER-α4, OR 1.18, 95% CI: 0.62-2.25, test for overall effect: Z=0.51, P=0.61; heterogeneity: χ2=15.47, P=0.004, I2=74%). No significant publication bias was observed by graph (Figure 3) or by Begg’s or Egger’s tests.
Discussion
The association of ER and PR expression with the EGFR mutation was controversial based on previous small-size reports. A meta-analysis is needed to incorporate all available results in order to give further insights on this conflicting issue. In the current literature, we found that high ER-β and PR expression was significantly higher, while ER-α to be high expression but the difference did not to be significance.
Adenocarcinoma is more likely to develop in women and young patients, which directs to the importance of estrogen in lung cancer. The preceding studies reported that an interaction of EGFR mutation and ER expression (including ER-α and nuclear ER-β), suggesting that some shared signaling pathway may exist between them (13). As acknowledged, there are several mechanisms to activate EGFR in lung cancer cells, including increased ligands and/or receptor, and mutation of the EGFR tyrosine kinase domain (2). It has been reported that the EGFR and mitogen-activated protein kinase 1 growth pathways will be activated when the rapidly released EGFR ligands in estrogen stimulation of lung cancer cells (13). Therefore, estrogenic signaling increased growth that depends on the EGFR pathway is rational.
In NSCLC cells, aromatase produces estrogen which stimulates the ER signaling pathway leading the development and progression of the tumor (13,20-24). It’s worth noting that the concentration of estradiol in NSCLC cells has a positively relationship with aromatase mRNA expression. Kohno and colleagues reported that cell line which has an EGFR mutation with a high aromatase mRNA expression was more sensitive to exemestane alone and cell growth was more significantly inhibited by the combination of exemestane and erlotinib than which has high aromatase expressions without EGFR mutations (20).
The previous studies have reported that estrogen down modulator could enhance antitumor activity in NSCLCs no matter alone or combined with EGFR-TKI. In our current study, high ER-α expression with EGFR mutation has no significant statistical difference with the control group. The reasons may be as follow. Firstly, different criterion exists in different studies: (I) antibodies and dilutions; (II) the subcellular location of ER-α; (III) scoring systems for staining. According to the report, ER-β only affect the lung adenocarcinomas with EGFR mutation suggesting that hormonal and EGFR pathways have a synergy in the progression of lung adenocarcinoma. In our study, the outcome of PR was controversial. It may associate with the small sample sizes. It was reported that PR was associated with better clinicopathologic features. For another, although ER-α showed 95% homologous identity to ER-β, immunohistochemical results show that ER-α mainly located in cytoplasm, while ER-β mainly located in nucleus in lung cancer cell and study showed that estrogen induce the proliferation mainly by ER-β.
This is the first study to comprehensively answer the interaction between EGFR mutation and ER. However, there are several limitations. First, exons identified as mutants were heterogeneous among included articles but we were unable to assess whether 19 or 21 exon alterations. In addition, we cannot assessment the progression-free survival (PFS) or overall survival (OS) for patients which have an EGFR mutation with a high estrogen expression.
In conclusion, high ER-β expression is correlated with EGFR mutations in NSCLC. The underlying mechanism and potential translational relevance warrant further investigation.
Acknowledgements
We want to give sincere thanks to all authors and patients of our included studies. We cannot complete this work without their work and participation.
Funding: Science and Technology Planning Project of Guangdong Province, China (Grant numbers: 2007B031515017; 2008A030201024); Science and Technology Planning Project of Guangzhou, China (Grant numbers: 2007Z1-E0111; 2007Z3-E0261).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Rouquette I, Lauwers-Cances V, Allera C, et al. Characteristics of lung cancer in women: importance of hormonal and growth factors. Lung Cancer 2012;76:280-5. [PubMed]
- Yano T, Miura N, Takenaka T, et al. Never-smoking nonsmall cell lung cancer as a separate entity: clinicopathologic features and survival. Cancer 2008;113:1012-8. [PubMed]
- Schwartz AG, Prysak GM, Murphy V, et al. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin Cancer Res 2005;11:7280-7. [PubMed]
- Kawai H, Ishii A, Washiya K, et al. Estrogen receptor alpha and beta are prognostic factors in non-small cell lung cancer. Clin Cancer Res 2005;11:5084-9. [PubMed]
- Ishibashi H, Suzuki T, Suzuki S, et al. Progesterone receptor in non-small cell lung cancer--a potent prognostic factor and possible target for endocrine therapy. Cancer Res 2005;65:6450-8. [PubMed]
- Márquez-Garbán DC, Chen HW, Fishbein MC, et al. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 2007;72:135-43. [PubMed]
- Skov BG, Fischer BM, Pappot H. Oestrogen receptor beta over expression in males with non-small cell lung cancer is associated with better survival. Lung Cancer 2008;59:88-94. [PubMed]
- Wu CT, Chang YL, Shih JY, et al. The significance of estrogen receptor beta in 301 surgically treated non-small cell lung cancers. J Thorac Cardiovasc Surg 2005;130:979-86. [PubMed]
- Sun HB, Zheng Y, Ou W, et al. Association between hormone receptor expression and epidermal growth factor receptor mutation in patients operated on for non-small cell lung cancer. Ann Thorac Surg 2011;91:1562-7. [PubMed]
- Chlebowski RT, Schwartz AG, Wakelee H, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet 2009;374:1243-51. [PubMed]
- Slatore CG, Chien JW, Au DH, et al. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J Clin Oncol 2010;28:1540-6. [PubMed]
- Stabile LP, Lyker JS, Gubish CT, et al. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res 2005;65:1459-70. [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed]
- Nose N, Sugio K, Oyama T, et al. Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol 2009;27:411-7. [PubMed]
- Raso MG, Behrens C, Herynk MH, et al. Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res 2009;15:5359-68. [PubMed]
- Toh CK, Ahmad B, Soong R, et al. Correlation between epidermal growth factor receptor mutations and expression of female hormone receptors in East-Asian lung adenocarcinomas. J Thorac Oncol 2010;5:17-22. [PubMed]
- Shimizu K, Hirami Y, Saisho S, et al. Membrane-bound estrogen receptor-α expression and epidermal growth factor receptor mutation are associated with a poor prognosis in lung adenocarcinoma patients. World J Surg Oncol 2012;10:141. [PubMed]
- Niikawa H, Suzuki T, Miki Y, et al. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res 2008;14:4417-26. [PubMed]
- Stabile LP, Davis AL, Gubish CT, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res 2002;62:2141-50. [PubMed]
- Pietras RJ, Márquez DC, Chen HW, et al. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids 2005;70:372-81. [PubMed]
- Mah V, Seligson DB, Li A, et al. Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res 2007;67:10484-90. [PubMed]
- Hershberger PA, Stabile LP, Kanterewicz B, et al. Estrogen receptor beta (ERbeta) subtype-specific ligands increase transcription, p44/p42 mitogen activated protein kinase (MAPK) activation and growth in human non-small cell lung cancer cells. J Steroid Biochem Mol Biol 2009;116:102-9. [PubMed]