Combination of cryosurgery and Iodine-125 seeds brachytherapy for lung cancer
Abstract
It has been proven that radioactive seeds such as Iodine-125 seeds implantation is a highly effective treatment for patients with localized cancer, such as lung cancer. It may increase the effectiveness of cryosurgery for lung cancer with the combination of Iodine-125 seed implantation into edge of the cryoablation zone. Percutaneous cryosurgery and Iodine-125 seed implantation are mutual complementation; both have been proved to be safe and effective modality for unresectable lung cancer, especially for centrally located lung cancer. Well-designed, randomized and control study both in the laboratory and in the clinical about this option are needed before the conclusive evidence submits.
Key words: Lung cancer; cryosurgery; cryoablation; Iodine-125 seeds
Introduction
External beam radiation (EBR) therapy is effective to many tumors, but the side effects are obvious and sometimes severe, limiting the repetitive use for the patient who has experienced radiation before. Therefore, brachytherapy has been adopted as a strategy to treat residual tumors. Brachytherapy has the ability to deliver a higher tumor dose of irradiation (70-90 Gy) compared to EBR, with good local control of the targeted tumor while sparing normal tissue outside the tumor. It is the most effective means of delivering conformal radiation and can be tailored to clinical circumstances (1).
It had been shown that Iodine-125 seed implantation is a highly effective treatment for patients with prostate cancer (2) and the head and neck cancers (3). Intraoperative lung and/or endobronchial brachytherapy in the management of non-small cell lung cancer offers a good curative potential in patients with accessible localized tumors, well defined and small to moderate in size, that have not metastasized to the lymph nodes and are technically or medically inoperable (1). Studies have also demonstrated that cryotherapy can increase the efficacy of radiotherapy because the residual tumor around the cryolesion exhibits a high metabolism and an enhanced vascularization, and cooled cells show more radiosensitivity (4,5). Moreover, the synergy between cryotherapy and radiotherapy has been documented (4,6). Therefore, it is plausible that the combination of cryotherapy and brachytherapy will increase the efficacy for the treatment of lung cancer.
Indication
Combination modality of cryosurgery and Iodine-125 seed implantation is suitable for the unresectable lung cancer and metastases:
-
- Centrally-located lung cancer that can not be covered by cryolesion;
- Large tumor that is difficult for cryosurgery to ablate all tumors;
- Localized small lymph node metastasis that is not suited for cryosurgery because of risks.
Technique
The procedure is performed either at the time of cryotherapy or one to two weeks after cryotherapy through the percutaneous approach under CT guidance (7). According to treatment planning system (TPS), the Iodine-125 seeds are implanted at the tumor border of cryotherapy or into the lymph nodules of the metastases. The number of seeds employed depends mainly on the tumor size, generally with each seed implanted at an interval of 0.5 cm or less.
Clinical data
Cryotherapy and radiotherapy
In a preliminary study, Vergnon et al. (4) investigated the efficacy of combined cryotherapy and irradiation for unresectable non-small cell lung cancer, and demonstrated that local control was obtained in 65% (17/26) of the patients with cryotherapy followed by irradiation compared with 35% with irradiation alone. In another report with radiotherapy 1 to 2 weeks after cryotherapy for advanced lung cancer, the 1- and 2-year survival rates were 70.2% and 53.1% compared to 42.1% and 20.0% with cryoablation alone (6). These results suggest that a combination of cryotherapy with radiotherapy achieve more benefit for the patients with lung cancer.
Cryotherapy and brachytherapy
Wang et al. (8) also evaluated the efficacy of percutaneous cryoablation combined with iodine-125 seed implantation in 51 patients with advanced lung cancer, and showed a reliable short-term effect with the total effective rate of 31.4%, 62.8%, 98.0%, and 92.0% at 1, 2, 3, and 6 months, respectively, after the treatment.
Wang et al. (9) evaluated the application, security and effectiveness of cryosurgery combined with radioactive seeds and release-controlled chemotherapeutic drugs implantation for percutaneous treatment of pulmonary neoplasma by CT guidance. Results showed the medium survive time was 16 months and average survive time was 14.0±2.6 months. More than 60% of patients survived more than 1 year.
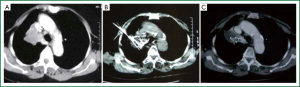
We (10,11) also investigated this mode of treatment for unresectable lung cancer. Fifty patients with non-small cell lung cancer NSCLC received the treatment for primary tumor and localized metastases of lymph nodes. Among them, 35 patients had centrally-located tumors. The result showed that 28 patients had tumor shrinkage (Figures 1,2) after the treatment. Thirty patients were followed-up for 12 to 41 months that the 1-, 2-, and 3-year survival rates was 68%, 43%, and 34%, respectively. Seven patients are alive more than 3 years. No obvious side effects were found. Later, we evaluated this mode of treatment in 140 patients with unresectable lung cancer and also showed a short-term survival improvement with 6-month and 1-year survival of 94.3% and 65.7%, respectively. Cough and dyspnea were improved in 51.7% and 59.8%, respectively. Again, no severe events happened.

Discussion
Endobronchial brachytherapy
High dose rate brachytherapy (HDRB) has several advantages such as less side effects and the treatment activity lasting longer compared with external beam radiotherapy (EBR), especially for the lung cancer patients who have been irradiated before (12). In addition, brachytherapy administered simultaneously with chemotherapy is better tolerated than a course of external beam irradiation and chemotherapy (1). This is especially worthful for the patients who have an indication for chemotherapy but have poorer performance status or other comorbidities.
Endobronchial brachytherapy (EBB) has been used to treat endobronchial obstruction caused by lung cancer that the response rate is 80% and is desirable for palliating these patients quickly without affecting quality of life. Furthermore, combination with regular external beam irradiation may increase the radiation dose and chance of cure (12).
In a prospective randomized study, Huber et al. (13) demonstrated the shorter treatment schedule (2×7.2 Gy with a 3-week interval) was more convenient for unresectable NSCLC patients with no more side effects and an equal local tumor control. Ozkok et al. (14) documented that It was safe and effective for the patients with stage III NSCLC to receive the combination treatment of external beam radiotherapy and high dose rate endobronchial brachytherapy or the patients with recurrent tumor who were irradiated previously with HDR-EB for palliative intent, the latter provides effective palliation in relieving the symptoms of patients with endobronchial lung cancer.
These results suggest that endobronchial brachytherapy is helpful to improve the symptoms of air way obstruction induced by lung cancer. The main life-threatening complications are hemoptysis and airway obstruction with an associated atelectasis and pneumonia. However, from a systematic review, Cardona et al. (15) concluded that EBR is more effective for palliation of NSCLC symptoms than EBB alone. In general, there was no evidence of benefit for EBB compared to EBR and Nd-YAG laser or for the combination of EBB with chemotherapy for the primary endpoint of survival. Nevertheless, for patients with recurrent endobronchial central obstruction but previously treated by EBR, EBB may be considered in selected cases.
Intrathoracic brachytherapy and lung resection
Brachytherapy has also been used as adjuvant therapy with Iodine-125 seed placement at the margins during limited lung resection. Combination of sublobar lung resection and Iodine-125 seed brachytherapy is gaining acceptance for T1 non-small cell lung cancer (NSCLC) in select patients whose comorbidities precluding lobectomy.
Lee et al. (16) found that after a limited resection of 35 primary non-small cell lung cancers with T1N0 and T2N0 in 32 patients who were not candidates for lobectomy or pneumonectomy, Iodine-125 brachytherapy seeds were implanted along the resection margin to reduce the risk of local recurrence. During the 20 to 98 months (median, 51 months) follow-up, the 5-year survival was 47% for all patients. The cancer-specific survivals were 77% and 53% for patients with T1N0 and T2N0 tumors, respectfully.
Voynov et al. (17) investigated another modality that after a limited surgical resection either by an open or video-assisted thoracoscopic procedure, a subsequent Iodine-125 Vicryl mesh brachytherapy implant to encompass a plane consisting of the staple line and a 2-cm margin of surrounding visceral pleura. Of the 110 patients with stage IA and IB NSCLC, the estimated 5-year local (in-field) control, locoregional control, and overall survival rates were 90%, 61%, and 18%, respectively.
Trombetta et al. (18) have investigated the efficacy and morbidity of surgical resection (segmental resection, wedge resection, or sublobar resection) and 125I impregnated Vicryl mesh brachytherapy placed in approximation to the aorta where 50% or greater of the implant volume directly approximated the aorta. The results showed the aorta could tolerate as high as 12,000 cGy interstitial Iodine-125 brachytherapy but supplemental, overlapping external beam irradiation should be avoided because one patient received this option suffered a fatal hemorrhage from suspected great vessel rupture.
There are few reports of percutaneous Iodine-125 seed implantation for lung cancer. Heelan et al. (19) reported seven lung cancer patients who received the treatment of percutaneous implantation of Iodine-125 seeds. No complications were encountered. Six patients were followed-up and evaluated by radiology that all the examination showed tumor shrinkage, and among them four cases shrank completely. These results suggest that Iodine-125 brachytherapy along a limited resection of lung cancer be a feasible procedure, result in a relatively low incidence of local recurrence and may prolong survival.
Pulmonary cryotherapy and brachytherapy
The main disadvantage of percutaneous cryosurgery is the incomplete ablation of peripheral margin of the cryoablation zone, especially in the case that the tumors close to large vessel. Implantation of Iodine-125 seeds into edge of cryoablation zone of lung cancer may be a complement of cryosurgery.
It has been proved that the patients who received this treatment are safe for people around them and the environment. They are not radioactive although within whose body contain iodine seeds, which is radioactive material. One of the benefits of iodine seeds is that almost all radiation stays within the tumor. After implantation, the patient can have physical contact with other adults as before.
It is feasible and safe for the Iodine-125 seed implanted into the tumor. From the above mentioned evidence of endobronchial brachytherapy and intraoperative brachytherapy, it is plausible that the combination of cryosurgery and Iodine-125 brachytherapy will be of promising approach for the patients with lung cancer.
Because percutaneous cryotherapy for lung cancer is widely performed in China, most data about this combination option come from Chinese literature (6-9,11). Results showed the patients received this approach gained more benefit in palliating the symptoms and overall survival compared with cryotherapy alone. However, the reported studies all have poor design. Patients were not randomizedly assigned, the assessors and the patients were not blinded to the treatment given. Moreover, the follow up was not regular among all patients. Therefore, the efficacy and safety of Iodine-125 seed implantation and cryotherapy for the treatment of lung cancer cannot be fully determined from the available evidence and needs well-designed, randomized and control study both in the laboratory and in the clinical.
Conclusions
Combination of cryosurgery and brachytherapy with Iodine-125 seeds provides a promising result for the patients with unresectable lung cancer, especially the patients with centrally-located tumors and mediastinal metastases. It seems related to a relatively low local recurrence and to improve survival of patients with lung cancer. However, well-designed, randomized and control study both in the laboratory and in the clinical about this option are needed before the conclusive evidence submits.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hilaris BS, Mastoras DA. Contemporary brachytherapy approaches in non-small-cell lung cancer. J Surg Oncol 1998;69:258-64.
- Merrick GS, Butler WM, Wallner KE, et al. Monotherapeutic brachytherapy for clinically organ-confined prostate cancer. W V Med J 2005;101:168-71.
- Mazeron JJ, Noël G, Simon JM, et al. Brachytherapy in head and neck cancers. Cancer Radiother 2003;7:62-72.
- Vergnon JM, Schmitt T, Alamartine E, et al. Initial combined cryotherapy and irradiation for unresectable non-small cell lung cancer. Preliminary results. Chest 1992;102:1436-40.
- Burton SA, Paljug WR, Kalnicki S, et al. Hypothermia-enhanced human tumor cell radiosensitivity. Cryobiology 1997;35:70-8.
- Yi FT, Song HZ, Chen HL, et al. Ar-He targeted cryoablation combined with radiotherapy in the treatment of advanced lung cancer. Shi Yong Ai Zheng Za Zhi 2004; 19:527-9.
- Niu L, Xu K, He W, et al. Percutaneous cryoablation for patients with advanced non-small cell lung cancer. Tech Cancer Res Treat 2007;6:451-2.
- Wang WH, Li FQ, Li L, et al. MSCT-guided percutaneous cryoablation combined with 125I-seed implantation for the treatment of lung cancer: an observation of short-term efficacy. Jie Ru Fang She Xue Za Zhi 2010;7:554-7.
- Wang H, Ma H, Luo L, et al. Cryosurgery combined with radioactive seeds and release-controlled chemical drugs implantation for the treatment of lung carcinoma. Zhongguo Fei Ai Za Zhi 2009;12:408-11.
- Xu KC, Niu LZ, Cryosurgery for Cancer. eds. Niu LZ, Zuo JS, Xu KC, et al. eds. Combination of cryosurgery and seed implantation for lung cancer. Shanghai: Shanghai Sci-Edu-Tech Pub, 2007:130-4.
- Zhou H, Niu L, Zhou L, et al. Cryosurgery combined with Iodine-125 seed implantation in the treatment of unresectable lung cancer. Zhongguo Fei Ai Za Zhi 2008;11:780-3.
- Kubaszewska M, Skowronek J, Chicheł A, et al. The use of high dose rate endobronchial brachytherapy to palliate symptomatic recurrence of previously irriadiated lung cancer. Neoplasma 2008;55:239-45.
- Huber RM, Fischer R, Haŭtmann H, et al. Palliative endobronchial brachytherapy for central lung tumors. A prospective, randomized comparison of two fractionation schedules. Chest 1995;107:463-70.
- Ozkok S, Karakoyun-Celik O, Goksel T, et al. High dose rate endobronchial brachytherapy in the management of lung cancer: response and toxicity evaluation in 158 patients. Lung Cancer 2008;62:326-33.
- Cardona AF, Reveiz L, Ospina EG, et al. Palliative endobronchial brachytherapy for non-small cell lung cancer. Cochrane Database Syst Rev 2008;2:CD004284.
- Lee W, Daly BD, DiPetrillo TA, et al. Limited resection for non-small cell lung cancer: observed local control with implantation of I-125 brachytherapy seeds. Ann Thorac Surg 2003;75:237-42; discussion 242-3.
- Voynov G, Heron DE, Lin CJ, et al. Intraoperative (125)I Vicryl mesh brachytherapy after sublobar resection for high-risk stage I non-small cell lung cancer. Brachytherapy 2005;4:278-85.
- Trombetta MG, Colonias A, Makishi D, et al. Tolerance of the aorta using intraoperative iodine-125 interstitial brachytherapy in cancer of the lung. Brachytherapy 2008;7:50-4.
- Heelan RT, Hilaris BS, Anderson LL, et al. Lung tumors: percutaneous implantation of I-125 sources with CT treatment planning. Radiology 1987;164:735-40.


