
The impact of income and education on lung cancer screening utilization, eligibility, and outcomes: a narrative review of socioeconomic disparities in lung cancer screening
Introduction
Rationale/background
Socioeconomic status (SES) is a complex entity commonly measured by income or education. SES reflects a person’s social standing and class, which greatly impacts all aspects of health (1-3). Those of low socioeconomic position have a shorter life expectancy and inferior quality of life (4-10). Among patients with non-small cell lung cancer (NSCLC), those of lower social class have higher cancer risk, incidence, and mortality (7,11-17). These inferior outcomes have been attributed to a higher likelihood of smoking and engaging in other risky behaviors, as well as lower access to quality healthcare, including treatment and clinical trial participation (18-27). Lower cancer screening rates also contribute to inferior outcomes among individuals of low socioeconomic position (28-30).
Most patients with lung cancer are diagnosed with stage IV disease (31,32). Lung cancer screening (LCS) is a life-saving tool used to discover lung cancer at an earlier stage when treatment can be curative. Early detection by LCS using low-dose chest computed tomography (LDCT) was associated with a 20% reduction in NSCLC mortality and a 6% reduction in all-cause mortality in the National Lung Screening Trial (NLST), a randomized controlled trial that compared the outcomes of smokers who were screened via LDCT vs. those who were screened via chest X-ray (33,34). Based on these results, the United States Preventive Services Task Force (USPSTF) developed LCS guidelines to reduce mortality from NSCLC, recommending annual screenings for 55–80-year-old individuals with a 30 pack-year smoking history or former heavy smokers who quit within the past 15 years (35-37). Subsequently, the Dutch-Belgian LCS [Nederlands–Leuvens Longkanker Screenings Onderzoek (NELSON)] randomized controlled trial (38-40), which included younger individuals with a lighter smoking history than the NLST, found an even greater mortality benefit of LCS. Based on these results, the USPSTF is updating its LCS guidelines to recommend annual screenings for 50–80-year-old current smokers with a 20 pack-year smoking history or former heavy smokers who quit within the past 15 years (41). This review was prompted by the recent change in the USPSTF guidelines. This narrative review aims to explore what additional changes may be needed based on additional factors contributing to LCS disparities.
Unfortunately, the studies that informed the LCS guidelines did not focus on recruiting socioeconomically diverse individuals, despite the inferior survival rates of those with fewer financial resources. In fact, the majority of the NLST study participants were of higher SES, and the percentage of participants with a college degree or higher (32%) was more than double the same percentage among individuals in the general population who met NLST age and smoking history inclusion criteria (14%) (34,42). Therefore, the study’s outcomes and the consequent guidelines may not be appropriate for those of lower socioeconomic position. Furthermore, LCS is significantly underutilized in general, and socioeconomic disparities likely exacerbate the barriers to LCS utilization (43). As such, a greater understanding of SES-based disparities in LCS utilization and outcomes is critical to achieving health equity in the NSCLC space.
Objective
The goal of this narrative review is to synthesize the results of the published studies that have examined the association between income and/or education and LCS. By evaluating current LCS practices and exploring the socioeconomic factors that impact screening, we can address the disparities in LCS and, ultimately, NSCLC outcomes. The key questions identified for the review topic include the following:
- How does SES, specifically education and income, affect LCS utilization?
- How does SES, specifically education and income, affect LCS eligibility?
- What is the influence that SES has on LCS outcomes?
- How should we move forward in understanding LCS through a SES lens?
We present the following article in accordance with the Narrative Review Reporting Checklist (available at: http://dx.doi.org/10.21037/jtd-20-3281).
Methods
Research selection
We worked with two professional medical librarians to search three online databases: PubMed, Ovid MEDLINE, and CINAHL Plus. We developed a list of index terms (Table 1) to find all publications from January 1, 2010, to October 21, 2020, that examined the impact of SES on LCS. Keywords included but were not limited to cancer screening, SES, education, income, lung cancer, and disparities.
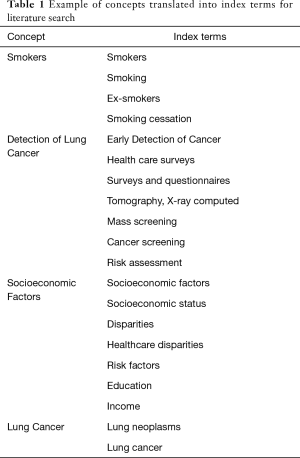
Full table
After removal of duplicates and an initial screening to ensure that articles fit the inclusion criteria, there were 70 articles remaining. Table 2 provides a list of inclusion and exclusion criteria for the articles used in this review. The title of each article was reviewed for topic relevance, and the abstract was reviewed for further clarification as necessary. Eight articles were then identified for inclusion in this narrative review. Data, including publication date, participant data, and study characteristics (author, title, study type, sample size, key findings, and outcomes), were abstracted from each article and placed in Table 3.
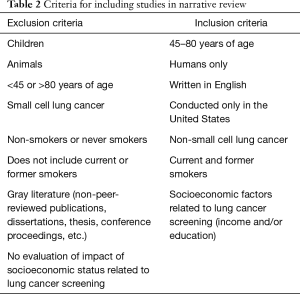
Full table
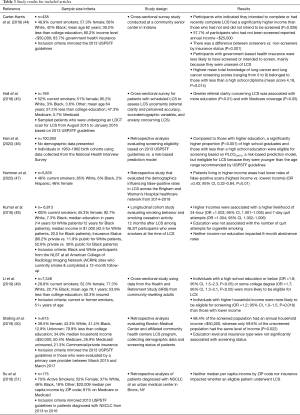
Full table
Discussion
SES influences LCS utilization
Only three studies evaluated the effects of SES-based factors on LCS utilization. Steiling et al. found that LCS rates are impacted by median income (50). The authors documented an overall screening rate of 16.1% among smokers evaluated at their institution who were eligible for LCS per the USPSTF LCS guidelines and found that, compared to individuals who were screened, those who were eligible but did not undergo screening were more likely to have a lower annual household income (50). Carter-Harris et al. noted the association of higher annual income with the completion of or intention to receive screening and found that those with government-based insurance also were less likely to complete or intend to complete screening (44). Su et al., however, found no significant differences based on race, ethnicity, median per capita income, or insurance type between patients who completed screening and those who did not (51). Their study evaluated 175 patients who were diagnosed with NSCLC between 2013 and 2016 and who were eligible for LCS based on USPSTF guidelines. Eighty-one percent of patients had Medicare or Medicaid insurance, with a median per capita income by home zip code (ZIP) of $20,009.
The discrepancy in these findings may be due to the different income measurements used in the three studies. Carter et al. gathered individual income data, whereas Steiling et al. gathered per capita income per ZIP and Su et al. gathered median household income per ZIP. These variables remind us that “income” means different things in different circumstances. Individual income reflects an individual’s SES, whereas ZIP/census-level income reflects the individuals’ neighborhood/community SES. Census-based measures of income are often used as proxies for individual-level income because the latter is not commonly available. However, although studies have revealed that individual-level and area-based income measures are significantly and independently associated with cancer risk, quality of life, and survival (52-56), the agreement between the two is frequently poor (56). Studies also suggest that low-income individuals have even lower outcomes and quality of life if they live in low-income neighborhoods. Therefore, the multi-level evaluation of SES allows for a more comprehensive understanding of the impact of SES on outcomes (53-55). It is also important to note that ZIP median household and ZIP per capita income cannot be used interchangeably. Unlike median household income, per capita income is based on the mean income of all individuals in a group. Because income has an important impact on health, we must use multiple, consistent metrics across studies to fully understand how our vulnerable patients are affected.
Lower LCS utilization among those with lower individual and median household incomes is likely secondary to multifaceted issues, including financial barriers. For instance, most low-income patients have either no insurance or government-based insurance. A low-income individual with no insurance would need to pay out-of-pocket for a scan, which is unlikely to happen due to various competing needs. And if an individual has Medicaid, they may not have full coverage of LDCT, as only 31 of 50 Medicaid fee-for-service programs cover LCS. Twelve state programs do not provide coverage, and 7 states did not have information available on their coverage (57). And although Medicare covers all LDCT screenings, not all high-risk individuals will qualify for such coverage due to age restrictions. Thus, insurance type and coverage can impact utilization rates in those with low SES.
In addition, individuals of low income are more likely to have jobs that are not flexible in allowing for LCS during work hours when radiology centers are typically open. Lastly, many patients of low income are cared for in clinics, including federally qualified health centers, that are under-resourced and lack specialists like pulmonologists. Providers at such locations have less time and support to perform shared decision-making, which is a required component of LCS (50). Data suggest primary care providers’ lack of time and knowledge about LCS impacts referrals for LCS (58-61). Certainly, lack of time and knowledge are more likely to be barriers in under-resourced clinics that low-income smokers tend to visit.
Hall et al. evaluated the impact that SES has on LCS uncertainty, including referral clarity and the perceived accuracy on screening. The study found that greater referral clarity about the reason for lung screening referral was associated with more education (P=0.01). Patients with Medicare also had decreased anxiety levels (P<0.01) and increased understanding of the purpose of LCS referrals (P<0.05) (45). The authors attributed these results to the Centers for Medicare and Medicaid Services’ efforts to provide eligible patients with information about LCS through routine flyers and an online portal. This study and others have emphasized that high educational attainment and health literacy can bolster a patient’s understanding of their treatment plan and, consequently, LCS rates (46,50,57). Williams et al. developed a 12-item measure of the pros and cons of getting screened for lung cancer in order to measure the decisional values of patients. The study found marginal associations between higher education levels and higher LCS knowledge scores (P=0.06) and an association between lower education levels and identifying greater cons in performing a LCS (P=0.09) (62).
The lack of patient clarity that is often associated with lower education can be addressed through the use of community educators or patient navigators. These team members play an important role in improving access and utilization of LCS among patients of lower SES status (45). Patient navigators impact the health of the underserved by facilitating access to the system by connecting patients to resources most appropriate for each patient’s individual needs. Navigators also provide advice regarding screening services that may improve compliance by increasing patients’ cancer knowledge and risk perception (61,62). Furthermore, they educate patients regarding screening guidelines which increases trust during shared decision making (56). Community educators and navigators assist with scheduling and transportation to screening centers, and with applying for insurance which are paramount to overcoming barriers to screening (63). Additionally, training community health workers can help increase LCS awareness by improving attitudes regarding screening benefits and reducing lung cancer stigma through education interventions (64). Many studies have shown that patient navigation improves cancer screening rates among underserved populations at community health centers by increasing utilization of screening (65,66) and follow-up after abnormal results (67), while decreasing disparities in care (68,69). Thus, high-risk patients of lower SES significantly benefit from patient navigator programs which help improve screening rates, compliance with follow-up, time to treatment initiation, patient satisfaction and quality of life (70).
Patient navigation is but one part of a required multifaceted approach to increase access and LCS utilization in the low SES community. The American Thoracic Society’s statement on addressing disparities in LCS eligibility and healthcare access revealed that Medicaid recipients are less likely to be asked about their smoking history, thus influencing their LCS utilization. The document also reinforced that the prevalence of tobacco smoking is highest among low-SES individuals, and the lack of coverage for certain Medicaid recipients leaves vulnerable at-risk populations without equitable access to screening. Moreover, individuals who smoke tend to be less educated and less likely to have a primary care provider, further reducing access to LCS (50). Therefore, obtaining an appropriate smoking history in those of low SES and providing adequate healthcare coverage to this group are necessary components to improving LCS utilization. To confront disparities in LCS faced by those of low SES, the multidisciplinary panel suggested using multilevel strategies, such as community outreach, education, telehealth, and patient navigation, to target barriers at the patient, provider, and healthcare system levels (50).
In summary, although individuals of low income are at higher risk for NSCLC, they face tremendous barriers to obtaining LCS. To help underserved groups overcome these barriers, we must increase their insurance coverage and could start by mandating that Medicaid universally cover LCS. Another useful strategy would be to increase the educational support available to smokers of low socioeconomic position and their providers. Health literacy is limited in individuals with lower income and education (71), so shared decision-making tools should also be geared toward those of low SES (61). The Agency for Healthcare Research and Quality (AHRQ) has recommendations on how to best provide health education to these vulnerable groups, including assuring that the decision aids are understandable and actionable. They also endorse the teach-back technique to increase the likelihood that patients understand the information (72). Additionally, incentives or support should be offered to providers serving these groups to ensure proper education and shared decision-making efforts (73).
SES influences LCS eligibility
Black smokers are diagnosed with NSCLC at a younger age, with fewer pack-years and shorter quit times than White smokers (74,75). Therefore, the 2013 USPSTF guidelines miss a significant number of high-risk, Black individuals. Low-SES smokers also have different smoking habits. These smokers tend to have a longer duration of smoking than those with higher income (24). They typically start smoking at a younger age and smoke more heavily (23). They are just as likely to make quit attempts but with less success than those with greater financial resources (27).
There have been important discussions regarding the associations between race-based disparities and LCS eligibility criteria (41,46,49,76-79). Risk-based models are more likely to identify high-risk Black smokers than the USPSTF 2013 guidelines. A handful of studies have reported SES-based disparities in eligibility as well. For instance, Han et al. evaluated the characteristics of younger (50–54-year-old) and older (71–80-year-old) smokers who were missed by the USPSTF guidelines but were identified as high-risk by the validated, risk-based PLCOm2012 screening model, which has been shown to be more sensitive than the USPSTF criteria. They found that, compared to those with higher education, a significantly higher proportion (P<0.001) of high school graduates and individuals with less than a high school education were ineligible for LCS because they were younger than the age range recommended by the USPSTF (46). In contrast, Li et al. found that, compared to those with a college education or higher, individuals with a high school education or less were more likely to be eligible for LCS (OR =1.8; 95% CI, 1.5–2.3). Interestingly, they also found that higher household income was associated with greater eligibility (49), suggesting like Han et al. that smokers with low SES are less likely to meet eligibility criteria.
The recent American Thoracic Society’s statement also highlighted the key fact that current LCS guidelines do not consider socioeconomic differences in smoking behaviors or lung cancer risk (50). The lack of incorporation of SES into LCS guidelines explains at least in part why the PLCOm2012 risk-based model, which incorporates comprehensive risk factors, including education, identified 12.4% more NSCLC cases, had fewer false positives, and had a higher positive predictive value compared to USPSTF criteria (80). These findings suggest that accounting for socioeconomic factors in guidelines may help increase the number of high-risk individuals eligible for screening. Recently, the American Gastroenterological Association published a white paper focused on the future of colorectal cancer screening. They too note that marginalized groups, including those of low income and those with less than high school education have increased barriers to obtaining colorectal cancer screening despite their higher risk of colorectal cancer. The authors describe strategies to decrease the barriers for these groups but the statement falls short of incorporating SES into their guidelines (81). Currently, there are no known cancer screening guidelines that include socioeconomic factors, but there are studies that are investigating the impact of including education in non-lung cancer risk prediction models (82). Mitigating existing SES-related disparities in LCS eligibility will require more studies specifically evaluating the suitability of current eligibility criteria for identifying at-risk individuals of lower socioeconomic position. Future studies should further evaluate the incorporation of SES into risk-based models, including individual and area-based income and education measures.
SES influences LCS outcomes
Our search identified only two articles evaluating the impact of SES on LCS outcomes.
False-positive rates
In order to determine which factors influenced the likelihood of a false positive LCS CT, Hammer et al. evaluated over 5,000 LCS scans that were performed across their healthcare network from 2014–2018. In the study, a false positive was defined by a Lung-RADS 3-4X (benign to suspicious categories) report with no diagnosis of lung cancer within 1 year. The authors found that false-positive rates were associated with many factors, including lower median income by ZIP (OR 0.43; 95% CI, 0.22–0.84, P=0.01) (57). Although the reasons for this association are not entirely clear, the authors proposed that it may arise because individuals of lower income tend to have worse overall health status and are at higher risk of infectious processes that simulate lung cancer. False-positive results could prompt interventions, such as biopsies, operations or additional imaging, which could lead to complications, resulting in even worse outcomes for low-income populations. Although invasive testing is very uncommon among individuals undergoing LCS (33), we recommend further studies investigating both the association between low SES and false positive rates and the link between SES and unnecessary invasive testing.
Smoking cessation
Only one study evaluated the impact of SES on smoking cessation rates among those who underwent LCS. The study found that individuals with higher self-reported household income were more likely to have 24-hour and 7-day quit attempts than those who reported lower household income (48). However, long-term abstinence was not impacted by income. Although smoking cessation rates in current smokers seeking LCS is universally low, nonetheless, the lower rate of smoking cessation attempts among low-SES individuals is concerning. A comprehensive focus on smoking cessation education and interventions may be especially important for low-SES patients. And PCP-focused educational efforts, incentives for complying with USPSTF recommendations, and measurable quality metrics on smoking cessation discussions and screening of high-risk patients may need to be implemented, especially in under-resourced communities where many underserved patients obtain care.
Intersectionality of Race and SES on LCS eligibility, utilization, and outcomes
Because race/ethnicity and SES are so tightly correlated, it is important to discuss the intersectionality of socioeconomic and racial disparities in LCS. Racial and ethnic minorities have the lowest SES. Several studies reveal that Black communities have lower eligibility and utilization of LCS (59,65,66) using current USPSTF guidelines. There are no studies that evaluate how low SES and minority racial status together impact LCS eligibility, utilization, or outcomes. Steiling et al. found that in a diverse community setting (41.4% Black individuals) Black participants had a lower screening rate, comprising only 37.6% of the screened population whereas White participants made up 46% of the screened population (P<0.001) (50). In the same sample, they found that unscreened patients had a lower annual household income than those who were screened. Certainly, we can imagine then that Black participants with a low annual household income would have an even lower screening rate than either individual group alone. And because data suggests that both low SES and racial minority status is also associated with lower eligibility, those who are both low SES and minority will likely have an even lower eligibility and worse outcomes. Therefore, investigation into the intersectionality between SES and race and their combined impact on LCS is paramount as we aim to improve outcomes in underserved groups.
Overall lung cancer patient survival has been significantly lower in more deprived neighborhoods, especially among lower SES, and ethnic-minority groups (75). These findings underline the importance of updating LCS guidelines to address both race-based and SES-based disparities in LCS eligibility, utilization, and outcomes.
Limitations of research reviewed
The main limitation to this review is the paucity of literature available on socioeconomic disparities on LCS. No studies identified in our search evaluated the impact of SES on several important outcomes, including annual adherence, stage of diagnosis at LCS, surgical rates, or mortality rates. Another limitation arises because the articles in this review utilized the 2013 USPSTF guidelines to screen participants instead of the upcoming guidelines and therefore it remains unclear how the new guidelines will impact patients of low socioeconomic position. In addition, as mentioned previously, there was no consistent SES metric across studies, so critical cross analysis was limited. Lastly, as with all narrative overviews, it is important to note that there are major differences between the studies presented (e.g., location, patient samples and research designs), thus we encourage readers to view how SES affects LCS in their own region.
Need for future research
In order to truly understand the impact of income and education on LCS, trials that intentionally recruit patients of low socioeconomic position are necessary. It is also important to explore how risk-based prediction models compare to the upcoming USPSTF guidelines and consider incorporating more SES measures into these models. Also, future studies need to analyze how lower individual and area-based education and income levels affect rates of follow up after positive findings (e.g., biopsies, PET and future CT scans), follow-up treatment (e.g., surgical resection and radiation treatment) and follow-up health outcomes (e.g., burden of disease and mortality rates).
Summary
This narrative review revealed several potential SES-related disparities in LCS screening rates, eligibility, and specific outcomes. Both income and education, key components of SES, have been associated with LCS eligibility, such that those of higher income are more likely to be eligible for LCS whereas those with lower education are more likely to be missed. Furthermore, among LCS-eligible patients, screening rates are lower among patients with lower income. Similar socioeconomic disparities persist beyond screening rates and eligibility, as well, as low-income populations experience fewer benefits from smoking cessation programs and may have higher false-positive rates from LCS.
Too few published studies have evaluated these associations to clearly define the mechanisms by which SES contributes to LCS disparities. Limited knowledge, time, and resources available to low-SES patients and their providers likely contribute to the LCS disparities they face. However, more research is needed to identify additional factors and to develop strategies to address them. Furthermore, as a result of the lack of relevant studies, it is not clear how SES impacts follow-up, stage at diagnosis, treatment, or mortality among LCS participants. Thus, additional studies evaluating the impact of individual income and education, as well as various area-based SES factors, on LCS eligibility, utilization, and outcomes are desperately needed. In the context of clinical practice, low-SES patients and their providers require further support, potentially through navigation and community health workers for patients or incentives and quality-metric evaluations for providers (83). These changes will ultimately help narrow gaps in NSCLC outcomes faced by low-SES populations.
Acknowledgments
Funding: This work was supported by the National Cancer Institute (NCI K12CA001727) and by a grant from AstraZeneca Pharmaceuticals LP.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Virginia Litle and Kei Suzuki) for the series “Socioeconomic Disparities in the Treatment of Thoracic Malignancies” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review Reporting Checklist. Available at: http://dx.doi.org/10.21037/jtd-20-3281
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3281). The series “ Socioeconomic Disparities in the Treatment of Thoracic Malignancies ” was commissioned by the editorial office without any funding or sponsorship. Dr. Raz reports receipt of an honorarium as a member of the advisory board for Roche. Dr Erhunmwunsee is the PI of a study supported by Astrazeneca Pharmaceuticals LP that seeks to improve lung cancer screening rates and education in underserved communities. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Measuring Socioeconomic Status and Subjective Social Status. American Psychological Association, Resources and Publication. 2015. https://www.apa.org/pi/ses/resources/class/measuring-status. Accessed October 8th 2020.
- Omer W, Al-Hadithi T. Developing a socioeconomic index for health research in Iraq. East Mediterr Health J 2017;23:670-7. [Crossref] [PubMed]
- Riaz SP, Horton M, Kang J, et al. Lung cancer incidence and survival in England: an analysis by socioeconomic deprivation and urbanization. J Thorac Oncol 2011;6:2005-10. [Crossref] [PubMed]
- Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic Disparities in Health in the United States: What the Patterns Tell Us. Am J Public Health 2010;100:S186-96. [Crossref] [PubMed]
- Countries NRCUPoUDTiLiH-I. The Role of Inequality. In: Crimmins EM PS, Cohen B, editor. Explaining Divergent Levels of Longevity in High-Income Countries. Washington (DC): National Academies Press (US), 2011.
- McDougall JA, Blair CK, Wiggins CL, et al. Socioeconomic disparities in health-related quality of life among colorectal cancer survivors. J Cancer Surviv 2019;13:459-67. [Crossref] [PubMed]
- Singh GK, Miller BA, Hankey BF. Changing Area Socioeconomic Patterns in U.S. Cancer Mortality, 1950–1998: Part II—Lung and Colorectal Cancers. J Natl Cancer Inst 2002;94:916-25. [Crossref] [PubMed]
- Tribius S, Meyer MS, Pflug C, et al. Socioeconomic status and quality of life in patients with locally advanced head and neck cancer. Strahlenther Onkol 2018;194:737-49. [Crossref] [PubMed]
- Zaninotto P, Batty GD, Stenholm S, et al. Socioeconomic Inequalities in Disability-free Life Expectancy in Older People from England and the United States: A Cross-national Population-Based Study. J Gerontol A Biol Sci Med Sci 2020;75:906-13. [Crossref] [PubMed]
- Ahmad TR, Tzou DT, Usawachintachit M, et al. Low Income and Nonwhite Race are Strongly Associated with Worse Quality of Life in Patients with Nephrolithiasis. J Urol 2019;202:119-24. [Crossref] [PubMed]
- Erhunmwunsee L, Joshi MB, Conlon DH, et al. Neighborhood-level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer 2012;118:5117-23. [Crossref] [PubMed]
- Cancer Trends Progress Report. National Cancer Institute, NIH, DHHS, Bethesda, MD March 2020.
- American Cancer Society. Key Statistics for Lung Cancer. 2020. Available online: https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html. Accessed April 15 2020.
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 2004;54:78-93. [Crossref] [PubMed]
- Greenwald HP, Polissar NL, Borgatta EF, et al. Social factors, treatment, and survival in early-stage non-small cell lung cancer. Am J Public Health 1998;88:1681-4. [Crossref] [PubMed]
- Tannenbaum SL, Koru-Sengul T, Zhao W, et al. Survival Disparities in Non–Small Cell Lung Cancer by Race, Ethnicity, and Socioeconomic Status. Cancer J 2014;20:237-45. [Crossref] [PubMed]
- Forrest LF, Adams J, Wareham H, et al. Socioeconomic Inequalities in Lung Cancer Treatment: Systematic Review and Meta-Analysis. PLoS Med 2013;10:e1001376 [Crossref] [PubMed]
- Pampel FC, Krueger PM, Denney JT. Socioeconomic Disparities in Health Behaviors. Annu Rev Sociol 2010;36:349-70. [Crossref] [PubMed]
- Becker G. Effects of being uninsured on ethnic minorities' management of chronic illness. West J Med 2001;175:19-23. [Crossref] [PubMed]
- Burstin HR, Lipsitz SR, Brennan TA. Socioeconomic Status and Risk for Substandard Medical Care. JAMA 1992;268:2383-7. [Crossref] [PubMed]
- Franks P, Clancy CM, Gold MR. Health insurance and mortality. Evidence from a national cohort. JAMA 1993;270:737-41. [Crossref] [PubMed]
- Ham DC, Przybeck T, Strickland JR, et al. Occupation and workplace policies predict smoking behaviors: analysis of national data from the current population survey. J Occup Environ Med 2011;53:1337-45. [Crossref] [PubMed]
- Siahpush M, Singh GK, Jones PR, et al. Racial/ethnic and socioeconomic variations in duration of smoking: results from 2003, 2006 and 2007 Tobacco Use Supplement of the Current Population Survey. J Public Health (Oxf) 2010;32:210-8. [Crossref] [PubMed]
- Tapan U, Furtado VF, Qureshi MM, et al. Racial and Other Healthcare Disparities in Patients with Extensive-Stage Small Cell Lung Cancer. JTO Clin Res Rep 2020:100109.
- Substance Abuse and Mental Health Services Administration. Results from the National Survey on Drug Use and Health: Detailed Tables. Rockville, MD, 2017.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA, 2014.
- Frederiksen BL, Jørgensen T, Brasso K, et al. Socioeconomic position and participation in colorectal cancer screening. Br J Cancer 2010;103:1496-501. [Crossref] [PubMed]
- Louwman WJ, van de Poll-Franse LV, Fracheboud J, et al. Impact of a programme of mass mammography screening for breast cancer on socio-economic variation in survival: a population-based study. Breast Cancer Res Treat 2007;105:369-75. [Crossref] [PubMed]
- Pruitt SL, Shim MJ, Mullen PD, et al. Association of area socioeconomic status and breast, cervical, and colorectal cancer screening: a systematic review. Cancer Epidemiol Biomarkers Prev 2009;18:2579-99. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [Crossref] [PubMed]
- Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax 2013;68:551. [Crossref] [PubMed]
- Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst 2010;102:1771-9. [Crossref] [PubMed]
- Pedersen JH, Ashraf H, Dirksen A, et al. The Danish Randomized Lung Cancer CT Screening Trial—Overall Design and Results of the Prevalence Round. J Thorac Oncol 2009;4:608-14. [Crossref] [PubMed]
- Swensen SJ, Jett JR, Sloan JA, et al. Screening for Lung Cancer with Low-Dose Spiral Computed Tomography. Am J Respir Crit Care Med 2002;165:508-13. [Crossref] [PubMed]
- Force USPST. Lung Cancer: Screening. U.S Preventive Services Task Force. 2020. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/lung-cancer-screening-2020. Accessed October 2020.
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- van Iersel CA, de Koning HJ, Draisma G, et al. Risk-based selection from the general population in a screening trial: Selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (73). Int J Cancer 2007;120:868-74. [Crossref] [PubMed]
- Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax 2017;72:48-56. [Crossref] [PubMed]
- Young K. Draft USPSTF Recommendations Expand Lung Cancer Screening. Available online: https://www.jwatch.org/fw116811/2020/07/07/draft-uspstf-recommendations-expand-lung-cancer-screening
- Tanner NT, Gebregziabher M, Hughes Halbert C, et al. Racial Differences in Outcomes within the National Lung Screening Trial. Implications for Widespread Implementation. Am J Respir Crit Care Med 2015;192:200-8. [Crossref] [PubMed]
- Jemal A, Fedewa SA. Lung Cancer Screening With Low-Dose Computed Tomography in the United States—2010 to 2015. JAMA Oncol 2017;3:1278-81. [Crossref] [PubMed]
- Carter-Harris L, Slaven JE Jr, Monahan PO, et al. Understanding lung cancer screening behavior: Racial, gender, and geographic differences among Indiana long-term smokers. Prev Med Rep 2018;10:49-54. [Crossref] [PubMed]
- Hall DL, Lennes IT, Carr A, et al. Lung Cancer Screening Uncertainty among Patients Undergoing LDCT. Am J Health Behav 2018;42:69-76. [Crossref] [PubMed]
- Han SS, Chow E, Ten Haaf K, et al. Disparities of national lung cancer screening guidelines in the U.S. population. J Natl Cancer Inst 2020;112:1136-42. [Crossref] [PubMed]
- Hammer MM, Byrne SC, Kong CY. Factors Influencing the False Positive Rate in CT Lung Cancer Screening. Acad Radiol 2020; [Crossref] [PubMed]
- Kumar P, Gareen IF, Lathan C, et al. Racial Differences in Tobacco Cessation and Treatment Usage After Lung Screening: An Examination of the National Lung Screening Trial. Oncologist 2016;21:40-9. [Crossref] [PubMed]
- Li CC, Matthews AK, Rywant MM, et al. Racial disparities in eligibility for low-dose computed tomography lung cancer screening among older adults with a history of smoking. Cancer Causes Control 2019;30:235-40. [Crossref] [PubMed]
- Steiling K, Loui T, Asokan S, et al. Age, Race, and Income are Associated with Lower Screening Rates at a Safety Net Hospital. Ann Thorac Surg 2020;109:1544-50. [Crossref] [PubMed]
- Su CT, Bhargava A, Shah CD, et al. Screening Patterns and Mortality Differences in Patients With Lung Cancer at an Urban Underserved Community. Clin Lung Cancer 2018;19:e767-73. [Crossref] [PubMed]
- Toubat O, Atay SM, Kim AW, et al. Disparities in Guideline-Concordant Treatment for Pathologic N1 Non-Small Cell Lung Cancer. Ann Thorac Surg 2020;109:1512-20. [Crossref] [PubMed]
- Myers V, Drory Y, Goldbourt U, et al. Multilevel socioeconomic status and incidence of frailty post myocardial infarction. Int J Cardiol 2014;170:338-43. [Crossref] [PubMed]
- Rocha V, Ribeiro AI, Severo M, et al. Neighbourhood socioeconomic deprivation and health-related quality of life: A multilevel analysis. PLoS One 2017;12:e0188736 [Crossref] [PubMed]
- Sanderson M, Coker AL, Perez A, et al. A multilevel analysis of socioeconomic status and prostate cancer risk. Ann Epidemiol 2006;16:901-7. [Crossref] [PubMed]
- Southern DA, McLaren L, Hawe P, et al. Individual-level and neighborhood-level income measures: agreement and association with outcomes in a cardiac disease cohort. Med Care 2005;43:1116-22. [Crossref] [PubMed]
- American Lung Asssociation and The University of Texas MD Anderson Cancer Center. Lung Cancer Screening Coverage in State Medicaid Fee-for-Service Programs. American Lung Association. 2020. Available online: https://www.lung.org/lung-health-diseases/lung-disease-lookup/lung-cancer/saved-by-the-scan/resources/state-lung-cancer-screening. Accessed October 27, 2020.
- Coughlin JM, Zang Y, Terranella S, et al. Understanding barriers to lung cancer screening in primary care. J Thorac Dis 2020;12:2536-44. [Crossref] [PubMed]
- Klabunde CN, Marcus PM, Silvestri GA, et al. U.S. primary care physicians' lung cancer screening beliefs and recommendations. Am J Prev Med 2010;39:411-20. [Crossref] [PubMed]
- Michaels M, D'Agostino TA, Blakeney N, et al. Impact of primary care provider knowledge, attitudes, and beliefs about cancer clinical trials: implications for referral, education and advocacy. J Cancer Educ 2015;30:152-7. [Crossref] [PubMed]
- Rivera MP, Katki HA, Tanner NT, et al. Addressing Disparities in Lung Cancer Screening Eligibility and Healthcare Access. An Official American Thoracic Society Statement. Am J Respir Crit Care Med 2020;202:e95-e112. [Crossref] [PubMed]
- Williams RM, Beck KH, Butler J 3rd, et al. Lung cancer screening decisional needs among African American smokers of lower socioeconomic status. Ethn Health 2020; Epub ahead of print. [Crossref] [PubMed]
- Natale-Pereira A, Enard KR, Nevarez L, et al. The role of patient navigators in eliminating health disparities. Cancer 2011;117:3543-52. [Crossref] [PubMed]
- Williams LB, Shelton BJ, Gomez ML, et al. Using Implementation Science to Disseminate a Lung Cancer Screening Education Intervention Through Community Health Workers. J Community Health 2021;46:165-73. [Crossref] [PubMed]
- Marshall JK, Mbah OM, Ford JG, et al. Effect of Patient Navigation on Breast Cancer Screening Among African American Medicare Beneficiaries: A Randomized Controlled Trial. J Gen Intern Med 2016;31:68-76. [Crossref] [PubMed]
- Percac-Lima S, Ashburner JM, Zai AH, et al. Patient Navigation for Comprehensive Cancer Screening in High-Risk Patients Using a Population-Based Health Information Technology System: A Randomized Clinical Trial. JAMA Intern Med 2016;176:930-7. [Crossref] [PubMed]
- Freund KM. Implementation of evidence-based patient navigation programs. Acta Oncol 2017;56:123-7. [Crossref] [PubMed]
- Percac-Lima S, Ashburner JM, Rigotti NA, et al. Patient navigation for lung cancer screening among current smokers in community health centers a randomized controlled trial. Cancer Med 2018;7:894-902. [Crossref] [PubMed]
- Percac-Lima S, López L, Ashburner JM, et al. The longitudinal impact of patient navigation on equity in colorectal cancer screening in a large primary care network. Cancer 2014;120:2025-31. [Crossref] [PubMed]
- Shusted CS, Barta JA, Lake M, et al. The Case for Patient Navigation in Lung Cancer Screening in Vulnerable Populations: A Systematic Review. Popul Health Manag 2019;22:347-61. [Crossref] [PubMed]
- Institute of Medicine Committee on Health L. In: Nielsen-Bohlman L, Panzer AM, Kindig DA, editors. Health Literacy: A Prescription to End Confusion. Washington (DC): National Academies Press (US), 2004.
- The SHARE Approach—Health Literacy and Shared Decisionmaking: A Reference Guide for Health Care Providers. Agency for Healthcare Research and Quality, Rockville, MD. 2014. Available online: https://www.ahrq.gov/health-literacy/professional-training/shared-decision/tool/resource-4.html#ref6. Accessed November 13th 2020.
- Raz DJ, Wu GX, Consunji M, et al. Perceptions and Utilization of Lung Cancer Screening Among Primary Care Physicians. J Thorac Oncol 2016;11:1856-62. [Crossref] [PubMed]
- Fiscella K, Winters P, Farah S, et al. Do Lung Cancer Eligibility Criteria Align with Risk among Blacks and Hispanics? PLoS One 2015;10:e0143789 [Crossref] [PubMed]
- Ebner PJ, Ding L, Kim AW, et al. The Effect of Socioeconomic Status on Treatment and Mortality in Non-Small Cell Lung Cancer Patients. Ann Thorac Surg 2020;109:225-32. [Crossref] [PubMed]
- Japuntich SJ, Krieger NH, Salvas AL, et al. Racial Disparities in Lung Cancer Screening: An Exploratory Investigation. J Natl Med Assoc 2018;110:424-7. [Crossref] [PubMed]
- Ryan BM. Differential eligibility of African American and European American lung cancer cases using LDCT screening guidelines. BMJ Open Respir Res 2016;3:e000166 [Crossref] [PubMed]
- Annangi S, Nutalapati S, Foreman MG, et al. Potential Racial Disparities Using Current Lung Cancer Screening Guidelines. J Racial Ethn Health Disparities 2019;6:22-6. [Crossref] [PubMed]
- Aldrich MC, Mercaldo SF, Sandler KL, et al. Evaluation of USPSTF Lung Cancer Screening Guidelines Among African American Adult Smokers. JAMA Oncol 2019;5:1318-24. [Crossref] [PubMed]
- Tammemägi MC, Church TR, Hocking WG, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med 2014;11:e1001764 [Crossref] [PubMed]
- Melson JE, Imperiale TF, Itzkowitz SH, et al. AGA White Paper: Roadmap for the Future of Colorectal Cancer Screening in the United States. Clin Gastroenterol Hepatol 2020;18:2667-78.e2. [Crossref] [PubMed]
- Jeon J, Du M, Schoen RE, et al. Determining Risk of Colorectal Cancer and Starting Age of Screening Based on Lifestyle, Environmental, and Genetic Factors. Gastroenterology 2018;154:2152-64.e19. [Crossref] [PubMed]
- Guide to Community Preventive Services. Cancer Screening: Multicomponent Interventions—Breast Cancer. 2020. Available online: https://www.thecommunityguide.org/findings/cancer-screening-multicomponent-interventions-breast-cancer. Accessed October 26 2020.


