Clinical validity of 25-gauge endobronchial ultrasound-guided transbronchial needle in lymph node staging of lung cancer
Introduction
Lung cancer is the leading cause of cancer incidence and mortality worldwide. Approximately 2.1 million new cases and 1.8 million deaths were reported in 2018, accounting for nearly 18.4% of all cancer-related deaths (1). The most important prognostic factor for lung cancer is disease stage, using the tumour-node-metastasis (TNM) classification of malignant tumours (2). Accurate staging can help reduce the rate of invasive procedures performed for patients unlikely to benefit from them and allows patients with curable disease to receive radical treatments, reducing the recurrence rate and improving the overall prognosis.
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a well-established procedure with a high diagnostic yield (3,4) and satisfactory safety profile (3,5); it is used in the differential diagnosis and lymph node (LN) staging of lung cancer (6). The other modalities recommended for staging are computed tomography (CT) and fluorodeoxyglucose positron emission tomography (FDG-PET); however, their diagnostic performance is insufficient, with a pooled sensitivity and specificity of CT estimated at 52–75% and 66–88% (7-10), respectively, and those of FDG-PET at 71–85% and 85–91% (11-14), respectively.
In contrast, EBUS-TBNA has a high diagnostic potential due to its capacity for making a pathological diagnosis with real-time guidance. In fact, the addition of EBUS-TBNA to the conventional combined CT and FDG-PET has been shown to improve diagnostic accuracy, with an estimated sensitivity and specificity of 90–93% and 99–100%, respectively (3,4,6). Based on this evidence, EBUS-TBNA has been recommended for LN staging (15). Accurate LN staging with EBUS-TBNA is increasingly important, as the prognosis of patients with unresectable stage III disease has improved significantly with chemoradiotherapy followed by durvalumab, an anti-programmed cell death ligand-1 antibody (16). Nevertheless, the standard 22-gauge (22-G) needle has some structural limitations, owing to its flexibility and penetrability; therefore, some stations approached at an acute angle are difficult to puncture (e.g., #2R/L, #3p, and #4L) (17,18). Meanwhile, a thinner 25-gauge (25-G) needle was introduced in Japan at the end of 2016. This needle offers improved flexibility and penetrability. Similar kinds of needles have been previously used in endoscopic ultrasound-guided fine needle aspiration (EUS-FNA), with a diagnostic accuracy comparable to that of standard 22-G needles (19). However, evidence for the usefulness of 25-G needles in EBUS-TBNA is limited (20), and previous studies have not reported on LN staging using the 25-G needle. Thus, we aimed to validate the clinical utility of this thinner 25-G needle in LN staging of lung cancer.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-3383).
Methods
This was a retrospective cohort study that included patients who underwent EBUS-TBNA for LN staging at our institution between November 2016 and March 2019. Cases that involved staging of previously confirmed or suspected primary lung cancer were included. Patients with histologically confirmed benign disease or other malignancies, patients with stage IV disease confirmed by other modalities, and patients who underwent re-staging after initial therapy for lung cancer were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The in-house National Cancer Centre Institutional Review Board (No. 2018- 090) approved the study and written consent for bronchoscopy was obtained from all participants.
Equipment and procedures
All procedures were performed with a convex probe-EBUS scope UC260FW (Olympus, Tokyo, Japan) and 25-G Expect™ Pulmonary Needle (Boston Scientific, MA, USA) by bronchoscopy experts or resident trainees under direct expert supervision. To make the patient comfortable during the procedure, moderate to deep sedation was maintained with a combination of intravenous narcotics (fentanyl or pethidine) and sedatives (propofol or midazolam). The bronchoscope was inserted via the oral route after topical aerosolised 8% lidocaine was applied; local intratracheal anaesthesia was performed with a 2% lidocaine injection.
Visual LN survey through EBUS-TBNA was performed in the order of N1, N2, and N3 stations. If the primary lesion was located along the central airway, it was assessed first. The risk of metastasis to each LN was systematically assessed with the following ultrasound modes. First, the B mode was used to assess the size, shape, margin, echogenicity, and the presence of a central hilar structure and a central necrosis sign (21). Second, the power/colour Doppler mode was used to assess the vascular patterns (22). Third, elastography was used to assess the relative stiffness of tissue when available (23). These features were identified during real-time evaluation.
Subsequently, LNs with echoic findings suggestive of malignancy (15,21) or at a location important to treatment protocols (such as N>2), depending on their metastatic status, were punctured in the order of N3, to N2, to N1 stations. If an unconfirmed primary lesion was noted, it was sampled last to prevent contamination.
Once the target LN or primary lesion had been identified, a needle with a stylet was inserted in real time into the target LN. After the puncture was performed and the needle tip was inside the tissue, the stylet was inserted beyond the tip to push out bronchial cells and minimise contamination. The stylet was then removed. The target LN was subjected to 20–30 needle strokes, while negative pressure was applied using a syringe to obtain tissue samples. However, in case of significant sample contamination with blood, the slow-pull technique was applied instead. Prior to retracting the needle into the sheath, the suction was removed to minimise the sample loss into the syringe (if used). Finally, the needle was removed from the bronchoscope to process the sample.
The tissues present within the needle were air-flushed into a slide glass for histologic examination. The needle was then flushed with normal saline solution to prevent clogging. The fluid used through this flushing was also collected using specimen containers for cytological examination. Wherever possible, the procedure was repeated at least twice to ensure sample adequacy. Each operator, depending on the results of the rapid on-site evaluation (ROSE), made decisions regarding sampling termination or addition. Moreover, for some LNs that were difficult to approach transbronchially, endoscopic ultrasound with bronchoscope-guided fine needle aspiration was also used.
Data collection and analysis
Data on patient age, sex, primary lesion lobe, punctured lesion, size of target lesions or LNs, number of needle passes, sampling rate, complications, and staging and pathology findings were extracted from the database at our institution. The genetic testing success rate was assessed.
The size of target lesions or LNs was measured by the short axis on a CT scan. The sampling rate was calculated as the ratio of number of passes core tissues could receive to that of total passes. After staging, some genetic testing was performed for patients who were eligible for chemotherapy or chemo-radiotherapy. The genetic testing included single-gene testing for targetable driver oncogenes, such as epidermal growth factor receptor mutation using the Cobas® EGFR mutation test (Roche Molecular Systems, CA, USA), and anaplastic lymphoma kinase (split fluorescence in situ hybridization assays). The success rate of genetic testing was calculated as the ratio of the number of samples with enough tissue for genetic testing to that of all samples. In patients with malignant LNs, determination was based on the cytology results of EBUS-TBNA with a subsequent clinical course consistent with that of malignant disease or with surgical-pathologic confirmation. In patients with benign LNs, determination was based on surgical-pathologic confirmation by complete thoracic lymphadenectomy or results of clinical follow-up of at least 12 months, demonstrating a lack of clinical or radiologic evidence of disease progression. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy were calculated. The diagnostic accuracy with and without EBUS-TBNA was compared based on the results of the combined CT and FDG-PET. The staging results of patients who underwent surgery were verified using resected specimens. Descriptive statistics were reported as counts, frequencies (percentages), and medians (ranges).
Results
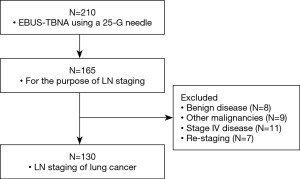
In total, 210 patients underwent EBUS-TBNA with the 25-G needle during the study period. Among them, 165 cases were eligible. Finally, the data of 130 patients were included in the analysis (shown in Figure 1).
Patient baseline characteristics and staging details are presented in Table 1. The median age was 69 [43–83] years, and most patients were male (71.5%). Most primary lesions were detected in the right lung (67.7%). A total of 349 lesions were sampled, and the median number of sampled locations per case was 3 [1–6]. The sampled locations varied with multiple stations (40.1%) in addition to stations #4R, #7, and #11, which were generally easy to puncture. The median size of the primary lesions was 23.1 (11.9–50.7) mm, while that of the LNs was 8.5 (3.2–40.8) mm.

Full table
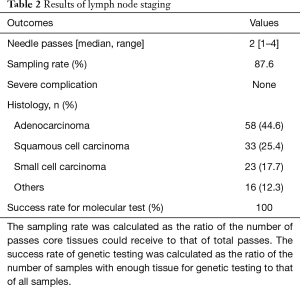
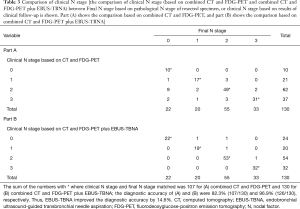
The LN staging results are presented in Table 2. The median number of needle passes per lesion was 2 [1–4], and the sampling rate was 87.6% (589/672 lesions). All LN staging procedures were successfully performed without any severe complications. The most frequent histological type was adenocarcinoma (44.6%), followed by squamous cell carcinoma (25.4%), small cell carcinoma (17.7%), and other types (12.3%). Of the 58 adenocarcinomas, 34 cases eligible for chemotherapy or chemo-radiotherapy were submitted to genetic testing, and completed in all cases (success rate 100%). The comparison of clinical N stage (based on combined CT and FDG-PET and combined CT and FDG-PET plus EBUS-TBNA) between Final N stage based on pathological N stage of resected specimen, or clinical N stage based on results of clinical follow up, is shown in Table 3. The diagnostic accuracy of combined CT and FDG-PET was 82.3%, and that of additional EBUS-TBNA was 96.9%. EBUS-TBNA improved the diagnostic accuracy by 14.6%.
Full table
Full table
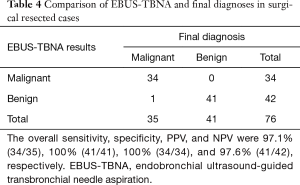
Finally, 40 cases (76 LNs) were pathologically verified using the specimens obtained during surgical resection (Table 4). The overall sensitivity, specificity, PPV, and NPV were 97.1% (34/35), 100% (41/41), 100% (34/34), and 97.6% (41/42), respectively.
Full table
Conclusions
This study validated the clinical utility of a 25-G needle in LN staging of lung cancer. To the best of our knowledge, this is the largest study on the clinical utility of EBUS-TBNA specifically for LN staging using the 25-G needle. The sampling rate and diagnostic yield obtained in this study were comparable to those obtained with traditional 22-G needles (3,4,6). However, a wide range of LNs, including those that were difficult to approach using 22-G needles (17,18), were aspirated using 25-G needles.
EBUS-TBNA is recommended for LN staging of lung cancer (6,15); however, the location of the tracheal or bronchial wall relative to target LNs can restrict needle access to these LNs. Although the combination of EBUS and EUS can help overcome this anatomical challenge (24-26), some LNs remain difficult to access, even by experts. This challenge is partially due to the structural limitations of standard 22-G needles. When 22-G needles with outer sheaths are inserted through the working channel of a bronchoscope, flexion of the tip is reduced due to the needles’ rigidity. LNs requiring a needle tip to bend for them to be punctured (for example, #2, #3p, #4L, and #10, among others) cannot be fully visualised when the contact with the tracheal or bronchial wall is lost. Jhun et al. reported that the diagnostic sensitivity and NPV of left-sided LNs were lower than those of midline or right-sided LNs (17). Gafoor et al. reported that #2 LNs were difficult to access due to limited flexion of the bronchoscope when the needle was applied (18). However, these authors achieved EBUS-TBNA for #2R with the 25-G needle, after failure with the 22-G needle. They speculated that a smaller diameter (1.52 mm) and specific needle composition (cobalt chromium) accounted for the improved flexibility of the 25-G needle.
In this study, in addition to stations #4R, #7, and #11, which are generally easy to puncture using conventional 22-G needles, multiple stations (40.1%) were punctured using the 25-G needle, enabling us to approach a wide range of LNs, owing to the needle’s flexibility. The 25-G needle has been used in EUS-FNA for abdominal lesions such as pancreatic masses before being introduced to EBUS-TBNA. The diagnostic yield and overall accuracy in EUS-FNA was comparable to those of the 22-G needle in a meta-analysis (19). Moreover, Camellini et al. reported that EUS-FNA was successfully performed using 25-G needles in masses at the uncinate process of the pancreas, which are difficult to puncture using the 22-G needle (27).
It was expected that needles as thin as 25-G may obtain smaller tissue samples and have a lower diagnostic yield than traditional 22-G needles. However, in this study, the sampling rate was 87.6%, and the diagnostic accuracy of the 25-G needle was 96.9% in all cases and 98.7% in surgically resected cases, suggesting a performance equivalent to that of 22-G needles (3,4,6). Furthermore, the LN staging revealed N2 and N3 stages in 54 and 32 cases, respectively; hence, 66.2% of the total cases showed progression, with mediastinal LN metastasis. Curative treatment such as surgery or radiation alone is not routinely recommended in these patients. Sufficient amounts of samples are needed for further genetic testing for an appropriate treatment choice (28). In this study, there was enough tissue for genetic testing in all samples, yielding a success rate of 100%. Stov et al. reported that next generation sequencing succeeded in 100% of cytology smear samples, with EBUS-TBNA using 25-G needles (29). This finding might be due to improved penetrability and sharpness of 25-G needles compared to those of 22-G needles. These features enable such needles to puncture relatively hard structures such as bronchial cartilage and lesions with calcifications. It has been suggested that omitting the use of stylet during EBUS-TBNA did not affect diagnostic outcomes and reduce procedural complexity (30). However, as the 25-G needle could easily puncture bronchial cartilage, the stylet was inserted beyond the tip, pushing out bronchial epithelial cells and minimising contamination in this study.
Studies on the use of 25-G needles in EBUS-TBNA are limited. Di Felice et al. reported that 25-G and 22-G needles achieved comparable specimen adequacy and diagnostic accuracy in EBUS-TBNA; however, their data were not specific to LN staging (20). In this study, EBUS-TBNA performed alongside the conventional combination of CT and FDG-PET improved the diagnostic accuracy by 14.6%. The desirable diagnostic accuracy of EBUS-TBNA of 96.9%, compared with the accuracy found in previous studies, suggests its clinical usefulness in lung cancer staging (31). In addition, although the frequency of complications of EBUS-TBNA using a 22-G needle is low (3,5), it is expected that the use of a 25-G needle will further reduce complications owing to its thinness (32). In the present study, no severe complications were observed, and we have had experience with EBUS-TBNA using a 25-G needle for diagnosis of hypervascular tumours such as metastatic lung tumour from renal cell carcinoma without any problems (33).
Despite its benefits, the use of the 25-G needle yielded some false negative findings in all cases (3.6%, 4/112) and surgically verified cases (2.9%, 1/35). Our study included a case, wherein an isolated tumour cell was confirmed in a resected specimen that was diagnosed as negative in preoperative EBUS-TBNA. Heterogeneous LNs that include both malignant and benign cells examined with several passes of EBUS-TBNA might yield false negative findings. However, it should be noted that the degree of sedation and number of needle passes were not prospectively standardised in this study; in fact, the degree of sedation might have been lighter, and the number of passes were lower than that recommended in early study stages (15). These factors might have affected our findings. Following standardisation, patients were kept under moderate to deep sedation and the number of needle passes was at least two, compliant with ROSE (34).
Another limitation of this study was that it was a single-centre retrospective study. Moreover, the performance of the 25-G needle was not directly compared with that of the 22-G needle, as this was a single-arm study. Owing to the usefulness of the 25-gauge needle in staging in clinical practice, we have been performing all staging with the 25-gauge needle since its introduction; thus, it was impossible to make a comparison in the same period. A randomised controlled trial is required to confirm the utility of the 25-G needle and the 22-G needle.
In conclusion, the 25-G needle enabled us to approach a wide range of LNs, with a satisfactory sampling rate and diagnostic accuracy in LN staging of lung cancer with EBUS-TBNA.
Acknowledgments
The authors would like thank Dr Adrian Christopher Lu for his insightful comments and Editage (
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-3383
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-3383
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3383). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The in-house National Cancer Centre Institutional Review Board (No. 2018- 090) approved the study and written consent for bronchoscopy was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Koh E, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer 2005;50:347-54. [Crossref] [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]
- Asano F, Aoe M, Ohsaki Y, et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: a nationwide survey by the Japan Society for Respiratory Endoscopy. Respir Res 2013;14:50. [Crossref] [PubMed]
- Dong X, Qiu X, Liu Q, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the mediastinal staging of non-small cell lung cancer: a meta-analysis. Ann Thorac Surg 2013;96:1502-7. [Crossref] [PubMed]
- Dales RE, Stark RM, Raman S. Computed tomography to stage lung cancer. Approaching a controversy using meta-analysis. Am Rev Respir Dis 1990;141:1096-101. [Crossref] [PubMed]
- McLoud TC, Bourgouin PM, Greenberg RW, et al. Bronchogenic carcinoma: analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology 1992;182:319-23. [Crossref] [PubMed]
- van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 2002;359:1388-93. [Crossref] [PubMed]
- Birim O, Kappetein AP, Stijnen T, et al. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg 2005;79:375-82. [Crossref] [PubMed]
- Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 2003;139:879-92. [Crossref] [PubMed]
- Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:178s-201s.
- Paul NS, Ley S, Metser U. Optimal imaging protocols for lung cancer staging: CT, PET, MR imaging, and the role of imaging. Radiol Clin North Am 2012;50:935-49. [Crossref] [PubMed]
- Wu Y, Li P, Zhang H, et al. Diagnostic value of fluorine 18 fluorodeoxyglucose positron emission tomography/computed tomography for the detection of metastases in non-small-cell lung cancer patients. Int J Cancer 2013;132:E37-47. [Crossref] [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical aspects of endobronchial ultrasound-guided transbronchial needle aspiration: CHEST Guideline and Expert Panel Report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Jhun BW, Park HY, Jeon K, et al. Nodal stations and diagnostic performances of endobronchial ultrasound-guided transbronchial needle aspiration in patients with non-small cell lung cancer. J Korean Med Sci 2012;27:46-51. [Crossref] [PubMed]
- Gafoor K, Belete H, Walczyszyn M, et al. Utility of a 25- versus 22-G EBUS needle in difficult-to-access 2R lymph nodes. J Bronchology Interv Pulmonol 2018;25:e17-9. [Crossref] [PubMed]
- Affolter KE, Schmidt RL, Matynia AP, et al. Needle size has only a limited effect on outcomes in EUS-guided fine needle aspiration: a systematic review and meta-analysis. Dig Dis Sci 2013;58:1026-34. [Crossref] [PubMed]
- Di Felice C, Young B, Matta M. Comparison of specimen adequacy and diagnostic accuracy of a 25-gauge and 22-gauge needle in endobronchial ultrasound-guided transbronchial needle aspiration. J Thorac Dis 2019;11:3643-49. [Crossref] [PubMed]
- Fujiwara T, Yasufuku K, Nakajima T, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest 2010;138:641-7. [Crossref] [PubMed]
- Nakajima T, Anayama T, Shingyoji M, et al. Vascular image patterns of lymph nodes for the prediction of metastatic disease during EBUS-TBNA for mediastinal staging of lung cancer. J Thorac Oncol 2012;7:1009-14. [Crossref] [PubMed]
- Izumo T, Sasada S, Chavez C, et al. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol 2014;44:956-62. [Crossref] [PubMed]
- Zhang R, Ying K, Shi L, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis. Eur J Cancer 2013;49:1860-7. [Crossref] [PubMed]
- Korevaar DA, Crombag LM, Cohen JF, et al. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: a systematic review and meta-analysis. Lancet Respir Med 2016;4:960-8. [Crossref] [PubMed]
- Labarca G, Aravena C, Ortega F, et al. Minimally invasive methods for staging in lung cancer: Systematic review and meta-analysis. Pulm Med 2016;2016:1024709 [Crossref] [PubMed]
- Camellini L, Carlinfante G, Azzolini F, et al. A randomized clinical trial comparing 22G and 25G needles in endoscopic ultrasound-guided fine-needle aspiration of solid lesions. Endoscopy 2011;43:709-15. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 6.2015. J Natl Compr Canc Netw 2015;13:515-24. [Crossref] [PubMed]
- Stoy SP, Segal JP, Mueller J, et al. Feasibility of endobronchial ultrasound-guided transbronchial needle aspiration cytology specimens for next generation sequencing in non-small-cell lung cancer. Clin Lung Cancer 2018;19:230-38.e2. [Crossref] [PubMed]
- Scholten EL, Semaan R, Illei P, et al. Stylet use does not improve diagnostic outcomes in endobronchial ultrasonographic transbronchial needle apiration: A randomized clinical trial. Chest 2017;151:636-42. [Crossref] [PubMed]
- Xu CC, Lei W, Jiang JH, et al. Endobronchial ultrasound-guided transbronchial needle aspiration can improve the diagnostic accuracy of positron emission tomography/computed tomography in hilar and/or mediastinal lymphadenopathy. J Cancer Res Ther 2019;15:1490-5. [Crossref] [PubMed]
- Lee JK, Lee KT, Choi ER, et al. A prospective, randomized trial comparing 25-gauge and 22-gauge needles for endoscopic ultrasound-guided fine needle aspiration of pancreatic masses. Scand J Gastroenterol 2013;48:752-7. [Crossref] [PubMed]
- Okubo Y, Matsumoto Y, Nakai T, et al. The new transbronchial diagnostic approach for the metastatic lung tumor from renal cell carcinoma-a case report. J Thorac Dis 2017;9:E762-6. [Crossref] [PubMed]
- Sehgal IS, Dhooria S, Aggarwal AN, et al. Impact of rapid on-site cytological evaluation (ROSE) on the diagnostic yield of transbronchial needle aspiration during mediastinal lymph node sampling: Systematic review and meta-analysis. Chest 2018;153:929-38. [Crossref] [PubMed]