A case of multiple pleural cryptococcosis without pleural effusion
Introduction
Pulmonary cryptococcosis can develop after inhalation of the fungus Cryptococcus neoformans. It tends to occur in immunocompromised hosts, although it can sometimes occur in immunocompetent individuals (1). The typical radiological manifestations include pulmonary parenchymal lesions, namely, pulmonary nodules, cavitary lesions, and consolidation (2). Thus, multiple pleural infiltrations secondary to pulmonary cryptococcosis, especially without pleural effusion, is extremely rare. Here, we report a case of multiple pleural cryptococcosis without pleural effusion.
Case report
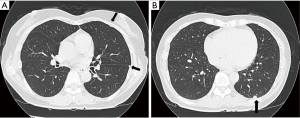
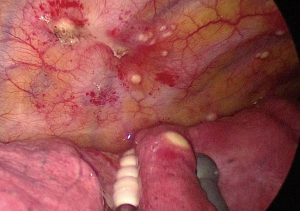
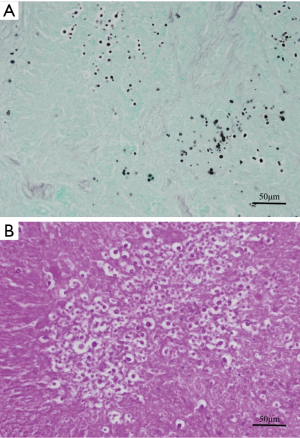
A 50-year-old woman who had no symptom was referred to our hospital for further evaluation of multiple left pleural nodules. Two years previously, the patient had undergone resection of stage II rectal cancer; at the time, histopathological examination of dissected para-aortic and pelvic lymph nodes also revealed follicular lymphoma. One year after surgery, the patient underwent 6 cycles of chemotherapy [R-CHOP; (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone)] because of progression of her malignant lymphoma. Severe pneumocystis pneumonia, a result of rituximab-related late-onset mild to moderate neutropenia (600-1,300/µL), occurred after the second cycle of chemotherapy; and she received trimethoprim-sulfamethoxazole combination therapy. After the full course of chemotherapy, chest computed tomography (CT) revealed multiple small, round nodules in her left lung. However, there was no uptake of 18fluorodeoxyglucose by any of the nodules on positron emission tomography. Serum level of tumor markers for rectal cancer (carcinoembryonic antigen, CA19-9) and malignant lymphoma (soluble IL-2 receptor) were within normal limits. There was no evidence of infection on routine laboratory testing; the white blood cell count, the percentage of neutrophils, and C-reactive protein level were within normal limits. Chest CT performed at our hospital showed multiple left pleural nodules without pleural effusion (Figure 1). Based on the clinical and radiologic findings, we made the presumptive diagnosis of pleural dissemination from lymphoma or rectal cancer. We considered trying non-invasive diagnostic method. However, all nodules were in the very peripheral lung or on the pleura, and therefore, bronchosopic biopsy was impossible. CT-guided transthoracic biopsy seemed to be very difficult because all nodules were very small. Finally, thoracoscopic exploration was performed for a definitive diagnosis. The intrathoracic findings showed multiple small, round, white nodules involving the visceral and parietal pleura, and similar intrapulmonary nodules in S10 (Figure 2). A lung wedge resection that included an S10 nodule and partial pleural biopsy were performed. The histopathological findings were consistent with pulmonary and pleural cryptococcosis (Figure 3). After the surgery, cryptococcal antigen was examined but it was negative. Although the patient did not have any neurological symptoms and any other findings of systemic dissemination, because serum level of β-D-Glucan was high (66.3 pg/mL), the patient received intravenous liposomal amphotericin B [150 mg (3.1 mg/kg/day)] for the first 2 weeks until denying systemic dissemination including central nervous system disease. During amphotericin treatment, the patient had no symptom and chest CT and X-ray showed that there was no interval change. And then, the patient received oral fluconazole (400 mg/day) for 7 months according to Clinical Practice Guidelines for the Management of Cryptococcal Disease (3). Recent chest CT showed all nodules shrank and serum level of β-D-Glucan was within the normal range.
Discussion
Pulmonary cryptococcosis is an uncommon fungal infectious disease that is usually caused by inhalation of Cryptococcus neoformans. It commonly occurs in immunocompromised hosts. Predisposing factors include acquired immunodeficiency syndrome, hematologic malignancies, organ transplantation, corticosteroid therapy, diabetes mellitus, and other conditions that impair T-cell mediated immunity (2). In particular, cryptococcosis is most commonly associated with AIDS patients. Investigators have reported that in Japan, the second most common underlying disease of pulmonary cryptococcosis is hematologic disease (3), probably because patients with hematologic malignancies are usually immunosuppressed as a result of antineoplastic therapy, including chemotherapy, targeted molecular therapy, and/or radiotherapy. Our patient underwent 6 cycles of R-CHOP chemotherapy for follicular lymphoma, with development of rituximab-related late-onset neutropenia, which could lead pleural cryptococcosis.
Pleural cryptococcosis, which is rare presentation, is generally accompanied by pleural effusion (4). Therefore, pleural cryptococcosis without pleural effusion is extremely rare, because, to the best of our knowledge, there have not been any previous reports of this condition. A possible explanation accounting for this clinical scenario might be that pulmonary cryptococcosis developed in the peripheral lung parenchyma of our patient during chemotherapy with a rupture into the intrathoracic space. Her quickly improved immune status after chemotherapy might have resulted in localized pulmonary cryptococcosis without development of empyema or systemic dissemination.
Distinguishing pulmonary and pleural cryptococcosis from thoracic malignancies by radiological findings is often difficult; there have been several reports that pulmonary cryptococcosis mimics lung cancer on imaging (5,6). The cryptococcal antigen test is a high sensitivity and specificity for diagnosis of cryptococcosis. However, sensitivity appears to be lower in HIV-negative and immunocompetent patients (7). Thoracoscopic surgery, which is a minimally invasive procedure, can obtain specimens that contain typical diagnostic lesion. In addition, thoracoscopy can be used to examine the intrathoracic space. Therefore, when multiple pleural nodules that do not manifest definitive diagnostic features are found on CT, thoracoscopic exploration and biopsy are probably the most effective procedures for obtaining the definitive diagnosis.
Conclusions
We reported a case of multiple pleural cryptococcosis without pleural effusion. It was a very rare presentation; however, clinicians should consider pleural cryptococcosis when a patient has pleural nodules without pleural effusion and a history of temporary immunosuppression even if cryptococcal antigen test is negative. Thoracoscopic exploration should be the best procedure for the definitive diagnosis of multiple pleural nodules.
Acknowledgements
Dr. Tanaka wrote the article, Dr. Murakami providing histopathological diagnosis, and other doctors helped proof reading the article.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Murayama S, Sakai S, Soeda H, et al. Pulmonary cryptococcosis in immunocompetent patients: HRCT characteristics. Clin Imaging 2004;28:191-5. [PubMed]
- Kishi K, Homma S, Kurosaki A, et al. Clinical features and high-resolution CT findings of pulmonary cryptococcosis in non-AIDS patients. Respir Med 2006;100:807-12. [PubMed]
- Kohno S, Kakeya H, Izumikawa K, et al. Clinical features of pulmonary cryptococcosis in non-HIV patients in Japan. J Infect Chemother 2015;21:23-30. [PubMed]
- Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis 2010;50:291-322. [PubMed]
- Newman TG, Soni A, Acaron S, et al. Pleural cryptococcosis in the acquired immune deficiency syndrome. Chest 1987;91:459-61. [PubMed]
- Igai H, Gotoh M, Yokomise H. Computed tomography (CT) and positron emission tomography with [18F]fluoro-2-deoxy-D-glucose (FDG-PET) images of pulmonary cryptococcosis mimicking lung cancer. Eur J Cardiothorac Surg 2006;30:837-9. [PubMed]
- Wang J, Ju HZ, Yang MF. Pulmonary cryptococcosis and cryptococcal osteomyelitis mimicking primary and metastatic lung cancer in (18)F-FDG PET/CT. Int J Infect Dis 2014;18:101-3. [PubMed]


