|
Original Article
Image-guided Radiotherapy Of Esophageal Cancer By Helical Tomotherapy:Acute Toxicity And Preliminary Clinical Outcome
Yi-Jen Chen, Kemp H. Kernstine, Stephen Shibata, Dean Lim, David D. Smith, Martin Tang, An Liu, Richard D. Pezner, Jeffrey Y. C. Wong
From Divisions of Radiation Oncology (Drs Chen, Liu, Pezner, and W ong, and Martin Tang) , Thoracic Surgery (Dr Kernstine), Medical Oncology (Drs Shibata and Lim), Biostatistics (Dr Smith), City of Hope Medical Center, Duarte, CA, USA
Corresponding to: Dr Yi-Jen Chen, MD, PhD, Division of Radiation Oncology, City of Hope National Medical Center, 1500 East Duarte Road, Duarte, CA 91010, USA. Tel: 626-301-8247; Fax: 626-930-5334. Email:yichen@coh.org
|
|
Abstract
Background:
Helical tomotherapy is a novel intensity-modulated radiotherapy modality with a helical 360° radiation delivery system and
CT imaging ability. The purpose of this report is to review our initial experiences and to assess the toxicity and efficacy of helical tomotherapy for esophageal cancer.
Methods:
Twenty patients with locally advanced esophageal cancer (T3-4 and/or N+ and/or M1a/b) were treated with helical tomotherapy.
Radiotherapy included simultaneous 50 Gy to gross tumorous areas and 45 Gy to areas of suspected subclinical disease. All received combination chemotherapy. Ten patients underwent surgical resection after completion of chemoradiation. Ten patients were ineligible for
surgery.
Results:
The treatment was well-tolerated. There were no treatment-related deaths or Grade 4 toxicity. Grade 3 toxicities were noted in 9 of
20 patients (45%). Down-staging was noted in 7 of 10 patients (70%) who underwent surgery. The median follow-up time was 24.5 months.
Eight patients, including 3 with surgery and 5 without surgery, have died. The 1-year overall survival rates for the entire group, patients with
and without surgical resection are 80.0%, 100.0% and 60.0% respectively (log-rank p = 0.244, surgery versus no surgery).
Conclusion:
The regimen of combined chemoradiation by helical tomotherapy for locally advanced esophageal cancer is well-tolerated.
The toxicity profile compares favorably with that of protocols based on conventional approach and the preliminary indications of efficacy
are encouraging.
Key words
Esophageal cancer; radiotherapy; Image-guided radiotherapy; helical tomotherapy.
J Thorac Dis 2009;1:11-16. DOI: 10.3978/j.issn.2072-1439.2009.12.01.013
|
|
Introduction
Radiotherapy (RT) plays a major role in multimodality
treatment for patients with esophageal cancer. The standard
therapy for patients with localized carcinoma of the esophagus
selected for nonsurgical treatment is combined chemoradiation up
to 50 Gy ( 1, 2). Delivering higher radiation doses did not increase
survival or local/regional control ( 3, 4). Adding surgery to
chemoradiation significantly increased local tumor control and
reduced chances of death from cancer ( 5). However, the results
were achieved at the cost of an in-hospital mortality rate of 11.3% in patients who underwent surgery. Therefore, studies focusing on
improving quality of treatment and reducing treatment-related
complications are essential.
Delivery of adequate radiation doses by the conventional
approach to the esophageal tumorous areas is limited by
radiation-sensitive normal structures in the thoracic cavity including
lungs, heart, and spinal cord. Helical tomotherapy is a novel RT
modality ( 6). It is a form of intensity-modulated RT (IMRT) that
uses a helic al 360° radiation delivery system. It delivers
image-guided RT through comparison of daily pretreatment
megavoltage CT (MVCT) scans with CT scans performed at the
time of simulation for treatment planning. By rapid opening and
closing of leaves in a collimator rotating around the patient,
tomotherapy prov ides the ability to sculpt radiation doses to
complex shaped tumor regions while limiting dose to normal
organs ( 7, 8). Compared to conventional IMRT techniques,
tomotherapy may provide sharper dose gradients around the target,
which will lead to superior sparing of surrounding normal structures
and possibly less radiation-related side effects ( 9- 11). Since October
2004, we have impl emented tom otherapy in the treatment of
patients with locally advanced, operabl e and non-operable,
esophageal cancer. The regimen includes upfront chemoradiation
up to definitive dose, 50 Gy ( 1, 2). The patients would then be
evaluated. For operable cases, including initially non-operablecases
who become operable after chemoradiation, surgical resection
would be offered. This report primarily evaluates the toxicity of
this regimen and provides preliminary efficacy data. To our
knowledge, this is the first study addressing the clinical efficacy
using helical tomotherapy for patients with locally advanced
esophageal cancer.
|
|
Patients and Methods
Patient population
Between October 2004 and January 2007, twenty consecutive
patients with locally advanced esophageal cancer treated with
chemoradiation using helical tomotherapy were identified. The
clinical data were collected and reviewed. This study was approved
by the Institutional Review Board of City of Hope Medical Center.
All cases had a minimum follow-up time from histological
diagnosis of 12 months. Workup prior to treatment included
esophagogastroduodenoscopy (EGD), CT scan of chest and
abdomen, endoscopic ultrasound (EUS) with fine needle aspiration
(FNA) if indicated, and PET scan. Twelve patients were
considered not surgical candidates initially because of T4/M1a/b
disease (10 cases) or severe co-morbidities (2 cases). After
completion of chemoradiation, the 5 cases with T4N1M0 disease were offered surgical intervention. Among these 5 cases, 3 went on
to undergo surgical resection and 2 patients refused the suggestion.
Patients with metastatic disease (M1a or M1b, 5 cases) and patients
with severe co-morbidities (2 cases) were not offered surgery after
chemoradiation. There were 8 initially operable cases and 1 did not
undergo surgery after chemoradiation because of patient refusal. In
summary, 10 patients completed upfront chemoradiation followed
by surgical resection and another 10 patients had chemoradiation
alone. Patient characteristics are shown in Table 1.
Chemotherapy
During radiation treatment, 15 patients received 2 cycles of
chemotherapy consisting of cisplatin 75 mg/m2 IV bolus on day 1
and 5-FU 1,000 mg/m2/24 hours infusion on days 1 through 4 of a
28-day-cycle chemotherapy. Two patients received continuous
5-FU without cisplatin because of renal insufficiency. Two patients
with severe co-morbidities received oral capecitabine 1,600 mg/m2
daily in two divided doses during RT. One patient received carbo
platin and paclitaxel. After completion of chemoradiation and/or
surgery, 10 patients received additional chemotherapy at the
discretion of treating physicians.
RT
Details of radiation treatment planning were described
previously ( 11). Briefly, prior to RT, a treatment-planning CT scan
was obtained. Based on diagnostic imaging, including EGD, EUS,
CT/PET scans, 2 target volumes were delineated. Gross tumor volume (GTV) consisted of areas with gross tumor. Clinical target
volume (CTV) consisted of areas with suspected subclinical disease
adjacent to GTV (an extension of 5 cm in the superior and inferior directions and 2 cm in the transversal direction) and celiac
nodal area for patients with lower thoracic esophageal carcinoma.
Margins were added to GTV and CTV to account for organ motion
and setup variations. An inverse IMRT plan was performed using
Tomotherapy Hi-Art system, version 2.0 (TomoTherapy, Madison,
WI). The prescribed dose was 50 Gy to GTV and 45 Gy to CTV in
25 fractions, which means both targets would be treated simultaneously with daily doses of 2.0 and 1.8 Gy respectively. The following inverse planning constraints were used: 95% coverage of the
targets to the prescribed dose, volume of lung receiving more than
10, 15 and 20 Gy (V10, V15 and V20) less than 40, 30 and 20%
respectively, volume of heart receiving more than 30 Gy (V30) less
than 30% and maximal dose to the spinal cord less than 45 Gy.
Surgery
Before surgery, patients were evaluated to ensure medical operability. Resection of the esophagus and the proximal stomach was
performed by a robotic-assisted minimally invasive approach
( 12, 13). Resection included excision of the paraesophageal, paracardial, left gastric, celiac and bilateral cervical lymph nodes. The
resected esophagus was replaced by the stomach with a cervical
esophagogastric anastomosis.
Toxicity A ssessment During Treatment and Follow-up
Patients were evaluated on a weekly basis during chemoradiation. Toxicity was scored using the National Cancer Institute's
Common Toxicity Criteria, version 2.0. Patients were evaluated
within 28 days after completion of all therapy. Follow-up assessments were performed every 3 months for 2 years, every 6 months
for 2 years, then yearly. For patients without surgery, imaging studies including CT scan, PET scan and EGD were done 3 months after completion of chemoradiation to evaluate response.
Statistical Methods
Survival and disease control parameters were calculated using
Kaplan-Meier analysis. Overall survival (OS) was defined as the
time from pathologic diagnosis until death or the last date of contact. Progression free survival (PFS) was defined as the time from
pathologic diagnosis until the date of disease recurrence or death.
We excluded patients with persistent disease from the PFS analysis. No patients were lost to follow-up.
|
|
Results
A cute Toxicity during chemoradiation
Treatment was well tolerated. Acute toxicity and weight change
are summarized in Table 2. There were no treatment-related deaths
or Grade 4 toxicity. Grade 3 toxicities were noted in 9 of 20 patients (45%). Twelve patients (60%) maintained stable weight (+/-
3% of initial weight) and among these, 3 actually gained 0.9, 1.0
and 1.2% of their initial weights respectively by the end of
chemoradiation. No patients required extra nutritional support,
such as enteral feeding or total parenteral nutrition.
Results for Patients Without Surgery
Among the 10 patients without surgery, studies at 3 months after chemoradiation confirmed 6 patients with complete response
including 1 case by FNA from a celiac node showing atypical cells.
Four patients were found to have partial response with persistent
disease.
Results at Surgery
Of the 10 patients who underwent surgery there were 9 R0 and
1 R1 resection. The mean number of examined lymph nodes was
21.7 (range, 10 to 39). Down-staging was found in 7 patients
(70%) by final pathology. No viable tumor was present in 2 specimens (20%). Four specimens (40%) showed microscopic residual
disease. Four patients were found to have persistent gross disease
including 1 patient with positive distant lymph nodes. Post-operative morbidity was seen in 6 cases (60%) including 5 anastomotic
leakages, 2 pneumonitis, 1 chylous effusion and 1 acute cholecystitis. No post-operative mortality was noted.
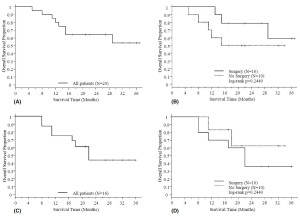
Survival
At the date of evaluation (February, 2008), 8 patients, including
3 with surgery and 5 without surgery, have died. The median follow-up time was 24.5 months.
The 1-year OS rates for the entire
group, patients with and without surgery were 80.0%, 100.0% and
60.0% respectively (log-rank p = 0.2440, surgery versus no surgery). The 2-year OS rates for the entire group, patients with
and without surgery were 64.3% , 78.8% and 50.0% respectively.
Excluding 4 patents with persistent disease by the end of treatment
in the chemoradiation only group, the 1-year PFS rates for the entire group, patients with and without surgery
were 75.0% , 70.0% and 83.3% respectively (log-rank p = 0.4279, surgery versus no
surgery). The 2-year PFS rates for the entire group, patients with
and without surgery were 43.7% , 36.0% and 62.5% respectively
( Table 3 and Fig. 1). Of note, we acknowledge that comparing results between patients with or without surgery after chemoradiation
is meaningless because of inhomogeneous population of patients
and obvious patient selection bias between the 2 groups.
|
|
Discussion
Definitive chemoradiation or chemoradiation followed by
surgery are two well-established curative treatments for patients
with locally advanced esophageal cancer
( 1, 2, 4, 5, 14- 16). For operable cases, compared to surgery alone, neoadjuvant chemoradiation and surgery usually improves overall survival and shows better
local-regional cancer control ( 15, 16). It is noted that neoadjuvant
chemoradiation also results in significant post-operative morbidity
and mortality ( 15, 17). Therefore, to reduce post-operative compli
cations, the radiation dose in neoadjuvant chemoradiation regimens
has been as low as 35 to 45 Gy ( 5, 17- 20). Unfortunately, oftentimes planned surgery is not done for various reasons. A study by
Stahl et al. has showed up to 34% of patients not proceeding to
surgery after neoadjuvant chemoradiation ( 5). Although these patients can receive additional chemoradiation later, the pause between two courses of radiation would cause repopulation of tumorous cells and hence inferior results ( 21). Therefore, better results
might be expected if upfront treatment up to 50 Gy could be delivered. However, higher postoperative complication rates could become an issue if surgery is going to be followed.
It is proven that lung sparing could be improved by IMRT compared to three-dimensional conformal RT (3DCRT) in treating
esophageal cancers ( 22, 23). Since the target volume of esophageal
cancer is approximately cylindrical and located at the center of the
body, the 360-degree freedom of beam projection of tomotherapy
is expected to provide more benefit in terms of treating esophageal
cancer. Indeed, compared to step-and-shoot IMRT, we have found
that tomotherapy can provide a preferred plan with better conformal
target coverage, more homogeneous target dose distribution
and better heart and lung sparing for patients with esophageal cancer ( 11).
In addition, with its image guidance ability by using daily
MVCT scan before each fraction of RT, setup errors could be detected and
corrected and thereby extra margins to account for setup
errors for target coverage could be reduced ( 24). Therefore, significant less amount
of lung and heart will be covered in radiation
treatment volume, which would likely reduce treatment-related
toxicities. This report summarizes our initial experiences of using
tomotherapy for patients with esophageal cancer. We were able to
deliver definitive/upfront RT dose up to 50 Gy without causing too
much toxicity. As shown in Table 2, the low toxicity profile of
chemoradiation by tomotherapy (45% grade 3 and no grades 4 or 5
toxicities) compares favorably with that of conventional approach,
66 to 76% grade 3 or higher toxicities ( 1, 4).
Studying patients undergoing neoadjuvant chemoradiation followed by surgery, Lee et al. have shown that the threshold for lung
irradiation for patients to be given multimodality therapy may be
lower than previously expected ( 25). By a multivariate analysis, a
lung V10 of 40% or more was the only factor that was associated
with occurrence of pulmonary complications. Using tomotherapy,
there is no postoperative mortality in our series. However, among
the 10 patients treated by combined chemoradiation followed by
surgery, 60% developed one or more severe complications, including 2 pneumonitis and 5 anastomotic leakages. Reviewing the
treatment planning for the two cases who developed pneumonitis a
lung V10 of 65% (in a patient with a long segment of disease) and
40% were identified respectively, indicating the importance of
minimizing the lung V10 as low as possible, perhaps <40% . The
50% anastomotic leakage rate in our series is higher than reported
by others. It is likely due to the fact that the greater curvature of the
gastric cardia used for cervical esophagogastrostomy anastomosis
was always covered by a moderate dose of RT by the nature of tomotherapy. To improve the results, we are conducting a project
with the surgeon to define the area of future anastomosis as an
avoidance structure in tomotherapy planning.
Locally advanced esophageal cancer is still a deadly disease.
With definitive chemoradiation up to 50 Gy, the RTOG 85-01 and
INT 0123 studies reported 50% and 68% 1-year survival rate respectively ( 1, 4). With combined chemoradiation followed by
surgery, trials by EORTC and University of Michigan reported
67% and 72% 1-year survival rates respectively ( 17, 19). It is noted
that the EORTC study included only stages I and II squamous cell
cancer and the study by University of Michigan included only potentially resectable cases. The 80.0% 1-year survival rate by our
regimen compares favorably to conventional approaches with or
without surgery. Since this is not a randomized study and because
of selection bias, we acknowledge that comparing survival between
patients with or without surgery after chemoradiation is meaningless although results showed slightly better survival for the group
of patients with surgery. It is not the intension of this paper to discuss the role of surgery after
chemoradiation. However, in fact, recently, the role of surgery after chemoradiation for responders has
been questioned. Study by Bedenne et al. has suggested that in patients with locally advanced esophageal cancer,
especially squamous cell carcinoma, responding to initial chemoradiation, there is
no benefit for the addition of surgery compared with continuation
of additional che moradiation ( 26). However, available clinical
prognostic factors do not help in choosing patients between responders and non-responders and therefore studies are still needed to search for new predictive factors and evaluate new tools to
detect early responders. Nevertheless, for operable locally advanced esophageal cancer including initially operable or cases becoming operable after chemoradiation treatment, routinely at our
institution we offer surgery following chemoradiation for feasible cases.
In conclusion, this is the first study evaluating patients with esophageal cancer treated with tomotherapy.
The toxicity profile compares favorably with that of protocols based on conventional RT approaches. The preliminary indications of efficacy are encouraging.
|
|
References
-
Herskovic A, Martz LK, Al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, et al.
Combined chemotherapy and radiotherapy compared with radiotherapy alone in
patients with cancer of the esophagus. N Engl J Med 1992;326:1593-8.
[LinkOut]
-
Cooper JS, Guo MD, Herskovic A, MacDonald JS, Martenson JA Jr, Al-Sarraf M,
et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). JAMA 1999;281:1623-7.
[LinkOut]
-
Gaspar LE, Winter K, Kocha WI, Coia LR, Herskovic A, Graham M. A phase I/II
study of external beam radiation, brachytherapy and concurrent chemotherapy for
patients with localized carcinoma of the esophagus (Radiation Therapy Oncology
Group Study 9207) final report. Cancer 2000;88:988-95.
[LinkOut]
-
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al.
INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74.
[LinkOut]
-
Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell
carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7.
[LinkOut]
-
Mackie TR, Holmes T, Swerdloff S, Reckwerdt P, Deasy JO, Yang J, et al. Tomotherapy: A new concept for the delivery of dynamic conformal radiotherapy.
Med Phys 1993;20:1709-19.
[LinkOut]
-
Mackie TR, Kapatoes J, Ruchala K, Lu W, Wu C, Olivera G, et al. Image guidance
for precision conformal radiotherapy. Int J Radiat Oncol Biol Phys 2003;56:89-105.
[LinkOut]
-
Balog J, Mackie TR, Pearson D, Hui S, Paliwal B, Jeraj R. Benchmarking beam
alignment for a clinical helical tomotherapy device. Med Phys 2003;30:1118-27.
[LinkOut]
-
Grigorov G, Kron T, Wong E, Chen J, Sollazzo J, Rodrigues G. Optimization of
helical tomotherapy treatment plans for prostate cancer. Phys Med Biol 2003;48:
1933-43.
[LinkOut]
-
van Vulpen M, Field C, Raaijmakers CP, Parliament MB, Terhaard CH, Mackenzie MA, et al. Comparing step-and-shoot IMRT with dynamic helical tomotherapy IMRT plans for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2005;62:1535-9.
[LinkOut]
-
Chen YJ, Liu A, Han C, Tsai PT, Schultheiss TE, Pezner RD, et al. Helical Tomotherapy for radiotherapy in esophageal cancer: a preferred plan with better
conformal target coverage and more homogeneous dose distribution. Med Dosim
2007;32:166-71.
[LinkOut]
-
Kernstine KH, Dearmond DT, Shamoun DM, Campos JH. The first series of completely robotic esophagectomies with three-field lymphadenectomy: initial experience. Surg Endosc 2007;21:2285-92.
[LinkOut]
-
Kernstine KH, DeArmond DT, Karimi M, Van Natta TL, Campos JH, Yoder MR,
et al. The robotic two-staged three-field esophagolymphadenectomy. J Thorac
Cardiovasc Surg 2004;127:1847-9.
[LinkOut]
-
Urschel JD, Vasan H, Blewett CJ. A meta-analysis of randomized controlled trials
that compared neoadjuvant chemotherapy and surgery to surgery alone for resectable esophageal cancer. Am J Surg 2002;183:274-9.
[LinkOut]
-
Urschel JD, Vasan H. A meta-analysis for randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable
esophageal cancer. Am J Surg 2003;185:538-43.
[LinkOut]
-
Fiorica F, Di Bona D, Schepis F, Licata A, Shahied L, Venturi A, et al. Preoperative chemoradiotherapy for oesophageal cancer: A systemic review and
meta-analysis. Gut 2004;53:925-30.
[LinkOut]
-
Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, et al. Chemora-
diotherapy followed by surgery compared with surgery alone in squamous-cell
cancer of the esophagus. N Engl J Med 1997;337:161-7.
[LinkOut]
-
Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7.
[LinkOut]
-
Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M.
Randomized trial of preoperative chemoradiation versus surgery alone in patients
with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305-13.
[LinkOut]
-
Burmeister BH, Smithers BM, Gebski V, Fitzqerald L, Simes RJ, Devitt P, et al.
Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomized controlled phase III trial. Lancet Oncol
2005;6:659-68.
[LinkOut]
-
Fowler JF, Lindstrom MJ. Loss of local control with prolongation in radiotherapy.
Int J Radiat Oncol Biol Phys 1992;23:457-67.
[LinkOut]
-
Chandra A, Guerrero TM, Liu HH, Tucker SL, Liao Z, Wang X, et al. Feasibility
of using intensity-modulated radiotherapy to improve lung sparing in treatment
planning for distal esophageal cancer. Radiother Oncol 2005;77:247-53.
[LinkOut]
-
Woudstra E, Heijmen BJM, Storchi PRM. Automated selection of beam orientations and segmented intensity-modulated radiotherapy (IMRT) for treatment of
oesophagus tumor. Radiother Oncol 2005;77:254-61.
[LinkOut]
-
Chen YJ, Han C, Liu A, Schultheiss TE, Kernstine KH, Shibata S, et al. Setup
variations in radiotherapy of esophageal cancer: evaluation by daily megavoltage
computed tomographic localization. Int J Radiat Oncol Biol Phys 2007;68:1537-45.
[LinkOut]
-
Lee HK, Vaporciyan AA, Cox JD, Tucker SL, Putnam JB Jr, Ajani JA, et al. Postoperative pulmonary complications after preoperative chemoradiation for
esophageal carcinoma: correlation with pulmonary dose-volume histogram parameters. Int J Radiat Oncol Biol Phys 2003;57:1317-22.
[LinkOut]
-
Bedenne L, Michel P, Bouche O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous
cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8.
[LinkOut]
Cite this article as: Chen YJ, Kernstine KH, Shibata S, Lim D, Smith DD, Tang M, Liu A, Pezner RD, Wong JY. Image-guided Radiotherapy Of Esophageal Cancer By Helical Tomotherapy: Acute Toxicity And Preliminary Clinical Outcome. J Thorac Dis 2009;1:11-16. doi: 10.3978/j.issn.2072-1439.2009.12.01.013
|