Surgical correction of 639 pectus excavatum cases via the Nuss procedure
Introduction
Pectus excavatum (PE) is the most common congenital chest wall deformity, accounting for over 90% of all chest wall deformities, with an incidence rate of approximately 0.1%, and a male to female ratio of 4:1 (1). Surgical correction is recommended because severe PE can affect the physical and psychological development of patients. In 1998, the American surgeon Donald Nuss, MD (2) first reported the successful repair of 45 cases of PE over 10 years via a minimally invasive surgical procedure without osteotomy. The Nuss procedure has the advantages of small and hidden incisions, short operative time, low blood loss, rapid recovery, and no need of cartilage or sternum resections. Through over 10 years of development, along with the improvement of surgical techniques and accumulation of experiences (3), the Nuss procedure has become the preferred and standard technique for the correction of PE. From September 2006 to August 2014, our department performed 639 Nuss procedures on patients with PE and achieved good short- to mid-term outcomes. This report presents the surgical technique essentials, efficacy, and postoperative complications.
Materials and methods
Clinical data
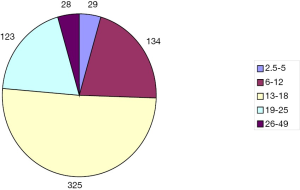
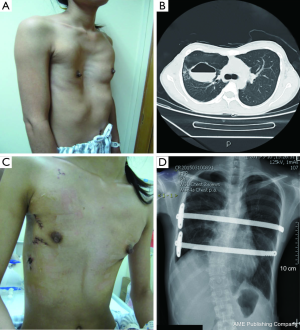
Our sample consisted of 639 cases, 546 males and 93 females. The patient’s ages ranged from 2.5 to 49 years, with a mean age of 15.3±5.8 years; among these, 29 were 2.5-5 years old, 134 were 6-12 years old, 325 were 13-18 years old, 123 aged 19-25, and 28 aged 26-49 (Figure 1). Fifteen patients presented with recurrent PE from previous operations, of which 8 underwent traditional procedures (6 sternocoastal elevations and 2 sternal turnovers) and 7 underwent the Nuss procedure. Two patients had previously undergone congenital heart surgeries, including 1 case of ventricular septal defect treated with transcatheter closure, 1 case of ventricular septal defect treated with closure surgery through median sternotomy, and 1 case who had previously undergone bronchial cystectomy surgery.
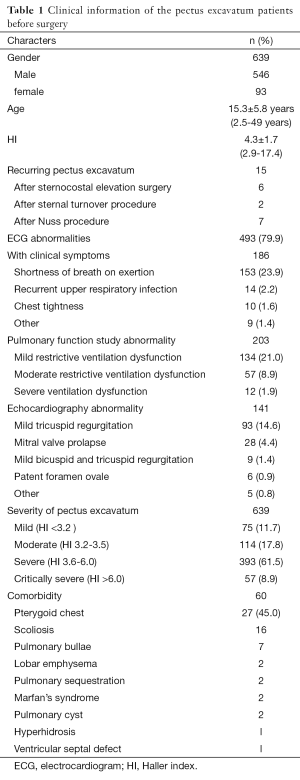
We collected a detailed medical history before each operation, performed a physical examination and electrocardiogram (ECG), echocardiogram, X-ray, chest CT and pulmonary function studies. Obvious preoperative clinical symptoms including shortness of breath on exertion, chest tightness, and recurrent respiratory infections were present in 186 cases (29.1%), while 493 cases (77.2%) showed ECG abnormalities, which included sinus arrhythmia, partial right bundle branch blockage, T wave irregularity, atrial or ventricular hypertrophy, intraventricular conduction delay, occasional atrial or ventricular premature contraction. A total of 223 (34.9%) had abnormal pulmonary function study results, and of these, 134 (21.0%) had mild restrictive ventilation dysfunction, 57 (8.9%) had moderate restrictive ventilation dysfunction, and 12 (1.9%) had severe ventilation dysfunction. A total of 141 (22.1%) patients had abnormal echocardiography results, with 93 (14.6%) presenting with mild tricuspid regurgitation and 28 (4.4%) with mitral valve prolapse. Patients were graded according to the Haller index (HI): mild, HI <3.2 (n=75, 11.7%); moderate, HI 3.2-3.5 (n=114, 17.8%); severe, HI 3.6-6.0 (n=393, 61.5%); extremely severe, HI >6.0 (n=57, 8.9%). Comorbidity were found in 60 (9.4%) patients, including 27 patients with pterygoid chest, 16 with scoliosis, 7 with pulmonary bullae, 2 with lobar emphysema, 2 with pulmonary sequestration, 1 with multiple atrial septal defect, 2 with clinically diagnosed Marfan’s syndrome, 2 with pulmonary cyst, and 1 with hyperhidrosis (Table 1).
Full table
Surgical indications
An operation is indicated if two or more of the following criteria apply (4): (I) chest CT shows HI >3.25; (II) pulmonary function study shows restrictive or obstructive lung disease; (III) ECG, echocardiography demonstrates incomplete right bundle branch blockade, valve prolapse and others; (IV) progression of the deformity with symptoms; (V) abnormal body image causing psychology problems; (VI) recurrent PE from previous surgery.
Surgical procedure
All patients were anesthetized with general intravenous anesthesia and underwent tracheal intubation. Previously selected Nuss steel bars (Walter Lorenz, US) were bent into a curved or convex shape to form the support frame. A transverse incision 2.0-2.5 cm long was made in each lateral chest wall between the anterior axillary and medial axillary lines, in line with the deepest point of the depression. After dissecting the subcutaneous tissues and muscles using an elecroscalpel, blunt dissection was carried out along the rib surface until reaching the deepest point using a finger. Then, a right pleural artificial pneumothorax was created with careful videothoracoscopic guidance, an introducer was inserted into the right thoracic cavity from the right lateral incision, advanced across the mediastinum immediately under the depressed sternum until it emerged on the left side. The surgeon and the assistant lifted the introducer, pressed the flared costarum several times using the right palm to shape it, inserted a 28 F chest tube via the introducer, inserted one end of the bent Nuss bar which has been shaped according to the patient’s chest contour, pulled back the chest tube so one end of the Nuss bar would emerge through the chest wall. The Nuss bar was then turned over, and the right side was secured through a fixation bar while the left side was sutured to the lateral chest wall muscles and rib periosteum. Fixation bars may be used on both sides in recurrent patients. The lung was extended and deflated before suturing the wounds in layers. Two bars can be used for patients with broad depression, the second bar was placed 2-4 cm below the deepest point of sternum. For severe asymmetric and unbalanced PE patients, personalized bars were used (Figure 2). In extremely severe PE patients, there is minimal space between the sternum and the vertebrate. In some cases, the deepest point of the sternum passes the anterior edge of the vertebrates. A small incision was made below the xiphoid to allow the use of fingers to guide the introducer below the sternum (Figure 3). To achieve better quality of reshaping, limited sternum or coastal cartilage resection may be performed if necessary.
Postoperative treatment
Epidural analgesia and antibiotics were given routinely 2-3 days postoperatively. Patients were encouraged to leave the bed as early as possible, but were reminded to keep the lower back straight and at shoulder level. Most of patients were discharged 5-7 days after the surgery and followed up regularly. Patients were instructed not to bend and rotate their bodies in one month. They were to avoid bending and moving heavy objects and perform strenuous exercise to prevent the displacement of the bar. Regular activities were permitted thereafter. They were seen and evaluated regularly. After 3 years or so, the bar was removed.
Evaluation of surgical outcome (2)
(I) Chest X-ray showed no sternum depression; (II) the morphology of chest wall is symmetry, without depression; (III) the patient and their families are satisfied; (IV) the thorax appears full with good extension and elasticity. The outcome is considered excellent if 4 criteria apply, good for 3, fair for 2 and poor for less than 1.
Results
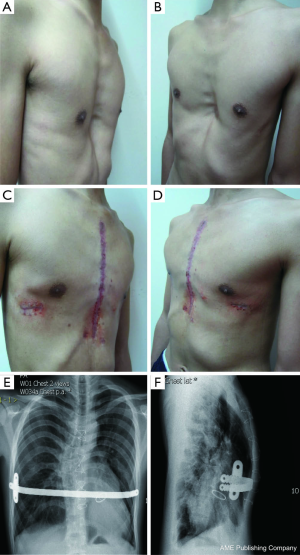
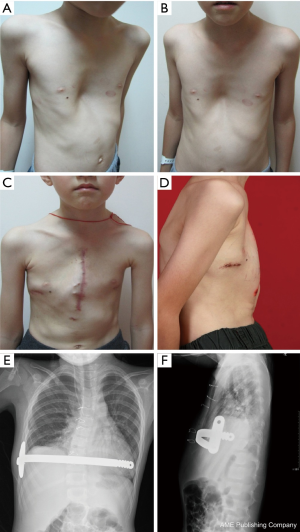
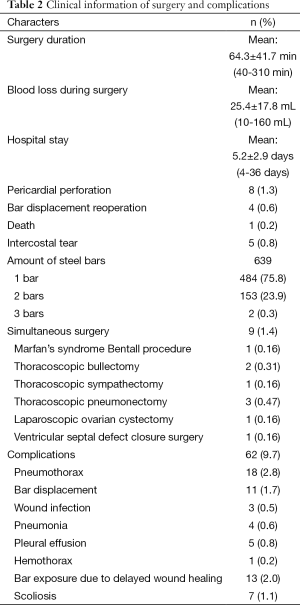
A total of 638 patients successfully completed the surgery, including 15 recurrent PE who underwent operation again (Figures 4,5). One patient died 17 days later due to a right atrial injury that occurred during the surgery. CT scan HI ranges from 2.9 to 17.4 (mean, 4.3±1.7). Operative durations varied from 40 to 310 minutes (mean, 64.3±41.7 min); besides the case of death, the blood loss ranged from 10 to 160 mL (mean, 24.5±17.8 mL). Postoperative hospitalization time ranged from 4 to 36 days (mean, 5.2±2.9 days). In 8 patients when pericardial perforation occurred, the introducer was withdrawn and replaced. In 5 patients, the intercostal muscles were torn when the bar slipped while being turned over, the incline bars replaced the intercostal or were fixed with additional wires. One patient with Marfan’s syndrome underwent atrial septal defect and ventricular septal defect closure, and replacement of the ascending aorta and aortic valve (Bentall operation) simultaneously (Figure 6). One patient underwent ventricular septal defect closure through median sternotomy incision (Figure 7). Two underwent bullectomy, 1 received thoracic sympathectomy surgery. One patient with pulmonary sequestration and 2 patients with pulmonary cyst underwent VATS pulmonary lobectomy (Figure 8). One patient underwent ovarian cystectomy. A total of 484 (75.7%) patients were treated with 1 bar, 153 (24.0%) received 2 bars and 2 (0.3%) received three bars. Fifty-four (39.7%) of the 136 patients who were older than 19 received more than 2 bars. Three patients with extremely severe PE and 1 PE after congenital heart disease surgery underwent additional incisions beneath the xiphoid, and 1 patient received 3 bars from left to right under surveillance of left-sided thoracoscopy.
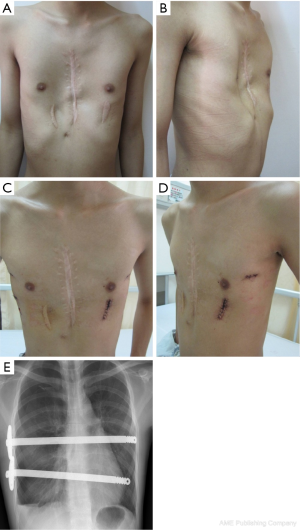
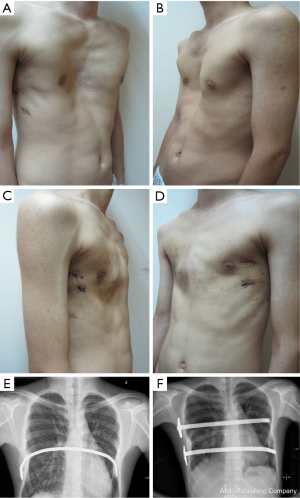
Postoperative complications: early pneumothorax occurred in 18 patients, but no treatment was needed. Right pleural effusion occurred in 5 patients, 3 underwent needle aspiration, 2 underwent percutaneous catheter drainage, and 450-1,500 mL of light bloody pleural fluid were drained. Hemothorax occurred in 1 patient, and it was resolved after percutaneous catheter drainage, blood transfusion and hemostatic therapy with 2,000 mL of bloody pleural fluid being drained. Pneumonia and atelectasis occurred in 4 patients and responded to antibiotics. Surgical site infection occurred in 3 patients, in one patient the right incision did not heal until the cross bar was removed 5 months later and treated with frequent dressing changes. Bar displacement occurred in 11 patients 1 month to 2 years after the surgery, 4 (displacement angle >15º) required reoperation and repositioning of the bar with an addition of a left-sided fixation bar. Wound fluid effusion and leakage occurred in 13 patients 5-23 months after surgery. Five resolved after wound opening and dressing change, 6 healed after debridement, in 1 patient, recurrent ulceration of right incision started from 6 months after surgery and resolved after the bar was removed 18 months later. The osteotomy wound infection occurred in 1 patient who received 3 bars and underwent sternal osteotomy. This patient was treated by using a transverse rectus abdominus myocutaneous flap to cover the wound. Mild acquired scoliosis occurred in 7 patients (Table 2).
Full table
The outcomes of the repair were excellent in 504 patients, good in 105, fair in 28, poor in 2; good quality rate was 95.3%. Patients were followed up at 1, 3, 6 months and 1 year after surgery. The mean follow up time is 44.0±27.9 months (1-98 months). A total of 304 patients already underwent bar removal, 3 patients had a few wire residual, with 1 undergoing reoperation to remove the wires, 1 receiving electrosurgical coagulation due to prolonged intercostal artery bleeding, and 1 receiving thoracotomy to stop internal thoracic artery bleeding. The average follow up time was 31.1±16.4 [5-77] months without any significant PE recurrence.
Discussion
The correction of PE via surgery has been performed for over a hundred years, with traditional surgical methods including sternocostal lifting and the sternal turnover method. However, traditional surgical methods have fallen out of favor with most modern patients and physicians because of the long operation time, massive muscle and cartilage damage, bleeding, trauma, slow recoveries, postoperative complications and high recurrence rates. In 1998, the American physician Donald Nuss (2) first reported the successful experience of surgically repairing PE using a minimally invasive method without osteotomy. The Nuss procedure involves a small and hidden incision, requires shorter operative times, results in less blood loss, allows for faster recoveries, does not require a chest wall muscle flap, does not require sternum cartilage or sternum resection, and can retain chest expansion, flexibility, and elasticity for a long period of time. Because of these advantages, its development is now commonly referred to as the revolution of chest wall orthopedic surgery. Along with the continual advance of the surgical techniques, asymmetric, recurrent PE as well as those with complications and those that occurred after congenital heart disease surgeries are also included in the scope of the Nuss procedure. The Nuss procedure has become the first choice and the standard procedure for the treatment of PE.
Currently, the ideal age for children to undergo the Nuss procedure for correction of PE remains controversial. Literature reviews show that the youngest patient that received a Nuss procedure was 1 year and 4 months old (5), and the oldest patient was 51 years old (6,7). Nuss believes that the ideal age to receive the repair procedure is between 6 and 12 years, his reasoning being that at this age, the deformity is localized to the costal cartilage and the involvement of the rib is minimal; therefore, after the surgery, the chest wall can develop normally. In addition, PE has a profound psychological impact on children that are affected, and therefore should be corrected before school age. For younger patients, it is difficult to manage the respiratory system after the operation, and they are not able to voluntarily expectoration, which makes them prone to respiratory complications. We typically choose patients over 6 years of age, although age is not an absolute determining factor. Factors such as cardiac and pulmonary dysfunction caused by PE, progressive symptoms and deformity may warrant the advance of the surgery. Our sample contained 24 cases (4.1%) under the age of 5 years, with the youngest patient being 2.5 years old. In recent years, more and more research studies (6,8,9) have reported that treatment of PE in adults via the Nuss procedure is also safe. Although there was a significant difference found in operative duration, postoperative pain intensity and duration between adult and youth patients, no significant difference was found in the complication and reconfiguration results between the two groups, and the procedure improves the quality of life for both groups of patients. Compared to youth recipients of the Nuss procedure, 19-37% (9-11) of adult PE patients required 2 metal bars implants, which is significantly higher than for youth patients. Over half of our patients were over 15 years of age, with an average age of 15.3 years, and a maximum age of 49 years. Among the 136 cases of adults 19 years older, 54 cases (39.7%) required more than one steel bar implant.
The complications of the Nuss procedure are divided into intraoperative and postoperative categories, with intraoperative complications primarily consisting of cardiac, pericardial, liver, diaphragm and intercostal vascular injuries. Cardiac injury is the most severe complication of the Nuss procedure (12-15), as an intraoperative injury of the heart or a major blood vessel could lead to intraoperative or postoperative mortality (12). All our patients underwent thoracoscopy to improve the safety of the Nuss procedure. Thoracoscopy could be inserted through the right or left chest to help accomplish the Nuss procedure. Although the heart is located on the left side, thoracoscopy from the left may not provide a good view, but in severe PE, insertion of the thoracoscope from left side could help observe the heart directly and maximally prevent the damage to the heart. Our experiences show that in recurrent PE patients or patients with lateral chest incisions, although posterior sternal adhesion is severe, the pericardium still remains intact. The use of assisted thoracoscopy can help bypass or burn the adhesive band; thus, preventing cardiac damage and reducing blood loss. For severe and complex cases of PE, an additional small incision under the xiphoid process may reduce the risk of cardiac damage. However, patients with congenital heart disease who have undergone previous median thoracotomy have a very high risk of heart injury from a secondary operation because of the defect in the pericardium and the fact that the heart and the sternum are attached tightly (12). In our case, the 11-year-old male patient who died after the surgery had undergone ventricular septal defect closure surgery through a sternal incision 7 years ago, although we created a small incision under the xiphoid process to separate the adhesion between the right atrium and sternum under the guidance of thoracoscopy, the right atrium was still damaged during the separation process, and although we repaired the right atrium via a sternotomy, the massive blood loss still resulted in severe hypoxic-ischemic encephalopathy, and the patient died 17 days later. Currently, we are collaborating on surgical procedures with cardiac surgeons for patients with congenital heart diseases comorbid with PE to attempt to perform the surgeries simultaneously to reduce the risk of cardiac injury from a secondary surgery for the correction of PE. In the meantime, we will implant an artificial pericardium to prevent the adhesion of the heart to the sternum after the operation to reduce the danger of cardiac injury during bar removal. One patient, who suffered from Marfan’s syndrome and PE, successfully underwent a simultaneous Bentall procedure. The most common complications of the Nuss procedure are pleural effusions, pneumothorax, bar displacement, wound infection and scoliosis and so on. Bar displacement is the major cause of surgical failures. Our experiences indicate that we should adjust the steel bar to the appropriate curvature for it to fit closely to the anterior chest wall. Therefore, we should chose a flat area on the sternum as the supporting point, and we need to protect the intercostal area where the bar enters or exits the chest wall to avoid tearing the intercostal muscles and use double fixation bars to fix the bar in patients with high risk of displacement. In patients with broader affected areas, we used 2 support bars and sutured the fixation bar on the steel bar with wires in the shape of an 8; then, we sutured the fixation bar to the rib periosteum together with chest wall muscles. This procedure reduces the dislocation rate significantly. No chest tube was placed routinely after the Nuss procedure, and postoperative pneumothorax was prevented by deflating the lungs and expanding the lungs before the incisions are sutured. Eighteen of our patients developed asymptotic pneumothorax, which required no special treatment and was possibly due to artificial pneumothorax during the surgery or inadequate expansion of the lung. However, pleural drainage tube is still recommended for the patients who undergo reoperation after recurrence or who have severe PE and receive multiple bars. One of our patients received 3 bars without placing a chest tube. He subsequently developed bilateral hemothoraces after the surgery and had to undergo several aspirations with 2,200 mL bloody pleural fluid being removed. Two other patients who received 2 bars developed hemothoraces after the surgery and were treated with percutaneous catheter drainage. When the introducer passes through the sternal gap, the feeling of resistance and the subsequent loss-of-resistance indicates the possibility of pericardial injury. At that time, the surgeon should withdraw the introducer, carefully observe and determine where the pericardium was perforated, bypass the pericardium and pass through the sternal gap and complete the procedure. Shin et al. (16) reported that most postoperative wound infections can be treated conservatively with debridement after removing the fixation bar when necessary. Usually, they do not need to remove the steel bar. With the gradual increase in the number of total cases of PE surgery and the accumulation of experience with such cases, the complication rate caused by surgeries has dropped significantly. However, because the number of cases of complex or recurrent PE is increasing and the difficulty of surgery is increasing, the risk of serious complications such as cardiac injury is rising as well.
Currently, the majority of PE patients who undergo the Nuss procedure have their steel bar implants removed 2-3 years after the surgery. Older patients and those with more severe or recurring cases of the disease may require an extended retention period. Under the following conditions, steel bar implants may be removed prematurely (17): if the patient cannot tolerate the pain caused by the steel bar implants, or if noticeable instances of scoliosis are caused by the pain from the steel bar implants; if there are severe allergies towards the material used in the steel implants and the patient experiences difficult wound healings; if the displacement of the metal bar implant causes exposure which leads to infection, and more conservative treatment methods fail to heal such infections; and if the dislocation of the steel bar implant affects the outcome of the reconfiguration. One of our patients experienced repeated ulceration of the left incision 6 months after the operation, conservative treatments were provided constantly until the bar was removed 18 months later and the wound healed afterwards. Thus far, 304 of our PE patients have undergone surgery for removal of the steel bar, most retaining the bars for approximately 3 years, and only 3 patients had small amounts of residual wires. There were no instances of major vascular and cardiac injury, or any other major surgical complications, and no significant recurrences occurred within the average 31.1 months follow-up. Short to mid-term effects of the surgery have proven satisfactory, while its long-term effects have yet to be observed.
The minimal invasive Nuss procedure is a relatively new technique, and there are not enough long-term follow up data available. However, as we accumulate experiences, and the surgical technique develops to become more and more sophisticated, studies will be performed to evaluate the postoperative and post bar removal outcomes and the improvement of life qualities (9,18,19). The effects of the procedure on PE patients’ cardiopulmonary function is also currently a matter of much concern (20-22). The optimal surgical procedure for each individual PE patients depending on their age and the type and degree of the deformity, is also a topic that requires further research and discussions.
Acknowledgements
Funding: This study was funded by the Guangdong Province Medical Scientific Research Foundation (A2015333 and B2010004) and Guangdong Province Science and Technology Planning Project (No. 20120318090).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Molik KA, Engum SA, Rescorla FJ, et al. Pectus excavatum repair: experience with standard and minimal invasive techniques. J Pediatr Surg 2001;36:324-8. [PubMed]
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998;33:545-52. [PubMed]
- Kelly RE, Goretsky MJ, Obermeyer R, et al. Twenty-one years of experience with minimally invasive repair of pectus excavatum by the nuss procedure in 1215 patients. Ann Surg 2010;252:1072-81. [PubMed]
- Kelly RE Jr. Pectus excavatum: historical background, clinical picture, preoperative evaluation and criteria for operation. Semin Pediatr Surg 2008;17:181-93. [PubMed]
- Park HJ, Lee SY, Lee CS, et al. The Nuss procedure for pectus excavatum: evolution of techniques and early results on 322 patients. Ann Thorac Surg 2004;77:289-95. [PubMed]
- Kim do H. Analysis of the Nuss procedure for pectus excavatum in different age groups. Ann Thorac Surg 2005;80:1073-7. [PubMed]
- Croitoru DP, Kelly RE Jr, Goretsky MJ, et al. Experience and modification update for the minimally invasive Nuss technique for pectus excavatum repair in 303 patients. J Pediatr Surg 2002;37:437-45. [PubMed]
- Coln D, Gunning T, Ramsay M, et al. Early experience with the Nuss minimally invasive correction of pectus excavatum in adults. World J Surg 2002;26:1217-21. [PubMed]
- Hanna WC, Ko MA, Blitz M, et al. Thoracoscopic Nuss Procedure for Young Adults With Pectus Excavatum: Excellent Midterm Results and Patient Satisfaction. Ann Thorac Surg 2013;96:1033-6; discussion 1037-8. [PubMed]
- Teh SH, Hanna AM, Pham TH, et al. Minimally invasive repair for pectus excavatum in adults. Ann Thorac Surg 2008;85:1914-8. [PubMed]
- Pilegaard HK, Licht PB. Early results following the Nuss operation for pectus excavatum-a single institution experience of 383 patients. Interact Cardiovasc Thorac Surg 2008;7:54-7. [PubMed]
- Schaarschmidt K, Lempe M, Schlesinger F, et al. Lessons learned from lethal cardiac injury by nuss repair of pectus excavatum in a 16-year-old. Ann Thorac Surg 2013;95:1793-5. [PubMed]
- Gips H, Zaitsev K, Hiss J. Cardiac perforation by a pectus bar after surgical correction of pectus excavatum: case report and review of the literature. Pediatr Surg Int 2008;24:617-20. [PubMed]
- Castellani C, Schalamon J, Saxena AK, et al. Early complications of the Nuss procedure for pectus excavatum: a prospective study. Pediatr Surg Int 2008;24:659-66. [PubMed]
- Bouchard S, Hong AR, Gilchrist BF, et al. Catastrophic cardiac injuries encountered during the minimally invasive repair of pectus excavatum. Semin Pediatr Surg 2009;18:66-72. [PubMed]
- Shin S, Goretsky MJ, Kelly RE Jr, et al. Infectious complications after the Nuss repair in a series of 863 patients. J Pediatr Surg 2007;42:87-92. [PubMed]
- Weih S, Reingruber B, Holland-Cunz S. Revision surgery in pectus excavatum after failure of Nuss technique. Thorac Cardiovasc Surg 2011;59:52-4. [PubMed]
- Kim HK, Shim JH, Choi KS, et al. The quality of life after bar removal in patients after the nuss procedure for pectus excavatum. World J Surg 2011;35:1656-61. [PubMed]
- Lam MW, Klassen AF, Montgomery CJ, et al. Quality-of-life outcomes after surgical correction of pectus excavatum: a comparison of the ravitch and nuss procedures. J Pediatr Surg 2008;43:819-25. [PubMed]
- Maagaard M, Tang M, Ringgaard S, et al. Normalized cardiopulmonary exercise function in patients with pectus excavatum three years after operation. Ann Thorac Surg 2013;96:272-8. [PubMed]
- Jayaramakrishnan K, Wotton R, Bradley A, et al. Does repair of pectus excavatum improve cardiopulmonary function? Interact Cardiovasc Thorac Surg 2013;16:865-70. [PubMed]
- Tang M, Nielson HH, Lesbo M, et al. Improved cardiopulmonary exercise function after modified Nuss operation for pectus excavatum. Eur J Cardiothorac Surg 2012;41:1063-7. [PubMed]