Convex probe endobronchial ultrasound: applications beyond conventional indications
Introduction
Clinical use of endobronchial ultrasound (EBUS) began in the 1990s (1). At the time, radial scanning was performed with the ultrasound (US) probe, which allowed for access deep into the airway through the working channel of the bronchoscope, thus obtaining ultrasonic tomography images of the surrounding tissues and structures outside the trachea and bronchus, but not real-time US guidance for transbronchial needle aspiration (TBNA). In 2002, convex probe (CP)-EBUS technology was introduced in clinical practice (2,3). The bronchoscope tip was equipped with an electronic convex array scanning ultrasonic probe. Such improvement allows for performing real-time EBUS-TBNA of enlarged hilar and mediastinal lymph nodes (4), which greatly improves the safety and accuracy of aspiration. Currently, EBUS-TBNA technology is maturing and gaining acceptance by more and more clinicians. Indeed, EBUS-TBNA has become a very important diagnostic tool for mediastinal diseases. Early-stage radiation probing has undergone gradual development, and is mainly used in the diagnosis of peripheral lung disease (5). Each of these EBUS techniques has unique characteristics and advantages. Herein we review the clinical application of CP-EBUS, excluding the literature pertaining to radiation probe EBUS.
The conventional indications for EBUS-TBNA mainly include lymph node staging for lung cancer and diagnosing mediastinal/hilar lesions (6-8). Current indications in details for EBUS-TBNA are described in the literature (9). Furthermore, the tumor samples obtained through aspiration can be fully used for genetic analysis, including EGFR and KRAS mutations (10). The aforementioned applications are the most common clinical applications for this technique. In addition, EBUS-TBNA has also been demonstrated to be an extremely safe procedure with an overall complication rate of <2% (11), which is the basis for the wide application of this technology. With the advent of EBUS-TBNA, endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has been partially replaced by EBUS (EUS-B-FNA) (12), while mediastinoscopy, a highly invasive examination, has also been largely replaced (13,14). Because the diagnosis of lung cancer lymph node staging by EBUS-TBNA is much more accurate than imaging examinations, such as positron emission tomography/computed tomography (PET/CT) (15), it has been suggested that routine EBUS-TBNA is suitable for patients with lung cancer as part of lymph node staging, instead of emphasizing the presence of iconographic, enlarged mediastinal lymph nodes (16). In fact, as a newly developed medical technology, the application of EBUS is not limited to the above indications. In clinical practice, many physicians have been using EBUS technology to evaluate and treat a number of diseases, including application of elastographic US imaging technology, diagnosis of pulmonary vascular diseases, treatment of mediastinum cysts and lymphangiomas. The current study reviewed the application of EBUS in other disciplines and for other indications, thus providing prospects for expanded applications in the future.
CP-EBUS for diagnosis
Elastography for mediastinal lesions
Ultrasonic technology includes many methods, including B- and Doppler US. When using EBUS to evaluate mediastinal lymph nodes, conventional B-mode US could be used to determine the size, shape, echoes, and borders of the lesion (17). At the same time, Doppler-mode US can be used in combination with B-mode US to analyze the blood flow of lesions (18), thus collectively determining the pathologic features of a disease. In recent years, elastographic technology has further promoted the clinical value of US imaging. The lesion, especially malignant lesions, is usually harder than the surrounding normal tissues, with less deformation under external forces of oppression, which has allowed for the development of elastographic techniques. Such technology can reflect the elastic properties of tissues, judging real-time hardness of the lesion, and facilitating the diagnosis of malignant lesions. Such technology was first applied in the field of breast US (19), and is also used for examination of the thyroid, prostate and liver (20-23). The endoscopic diagnosis of benign or malignant pancreatic diseases using elastography has been demonstrated with very high sensitivity and accuracy (24-26). Currently, the application of elastographic techniques in EBUS has become a reality, which allows elastographic imaging of mediastinal lymph nodes due to intermittent pressure on surrounding tissues by the aorta and pulsating heart. Researchers have begun to determine the malignant features of mediastinal lymph nodes using elastography. Using a small sample size (ten patients with 13 lymph node areas measuring 10-30 mm), Trosini-Désert et al. (27) showed that elastography in EBUS is feasible and bronchial tracheal cartilage does not affect the collection of elastic data. Although the sample size was small, the study of Trosini-Désert et al. (27) concluded that the introduction of elastography improved the diagnostic ability of EBUS. Since then, Izumo et al. (28) evaluated 75 lymph nodes using EBUS elastography. The elastographic results of lymph nodes were divided into three types, as follows: type 1, predominantly non-blue (green, yellow, and red); type 2, part blue, part non-blue (green, yellow, and red); and type 3, predominantly blue. Then, according to the comparison between the pathologic and elastographic results, type 1 lymph nodes were benign (24/24, 100%), type 2 lymph nodes were mixed benign and malignant, while the malignant percentage of type 3 lymph nodes was >90% (35/37, 94.6%). For type 1 (benign) and 3 (malignant) lymph nodes, the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy rates were 100%, 92.3%, 94.6%, 100% and 96.7%, respectively.
The above results indicated that elastography is very accurate in predicting the properties of types 1 and 3 lymph nodes, but not in type 2, suggesting that this technology has limited value, and studies with a larger sample size are needed. If the results with a larger sample size support such findings, then elastographic results could be used to determine whether or not the targeted lymph node aspiration should be performed. For type 1 lymph nodes, aspiration could be bypassed. But with type 3 lymph nodes, even if the size of the lymph node is very small, aspiration should be carried out to avoid underestimation of tumor lymph node staging. For type 2 lymph nodes, more specific aspiration of the blue region of the lymph nodes under the guidance of elastographic US should be considered. Because the distribution of tumor cells in lymph nodes may not be even, the hardness of the same lymph nodes could vary in different regions, with a greater possibility of obtaining tumor cells in the blue region than the green or red region, which is inconclusive and warrants further study.
Elastographic imaging can demonstrate lesion stiffness, and even demonstrate the scale of lesions and infiltration to surrounding tissues. It was concluded based on a recent case report (29) that the degree of infiltration of tracheal tumors to the esophagus can be judged effectively by elastographic imaging.
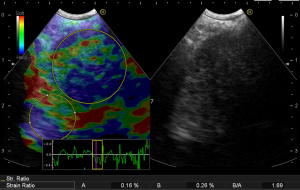
In the process of performing elastography, the strain ratio can also be measured, which is the ratio (B/A) between the average elastic hardness of the lesion (A) and the normal region (B), and calculated with software of the ultrasonic equipment. The strain ratio is a method to quantify the hardness of the lesions, which allows determination of benign or malignancy status based on numerical data, instead of estimation and classification solely based on color. Although research data in this area is very limited, quantification is the inevitable trend with respect to the further development of elastography. Currently, standardization of such quantification is the biggest problem restricting its application. The general principle is that the A zone should cover the entire lesion area as much as possible, and a non-diseased area with a variety of colors mixed should be selected as the B zone (Figure 1). According to such a standard, the area selected by different operators would be different, resulting in different ratios. Therefore, the standardization method is an urgent problem in need of resolution.
Pulmonary embolism (PE)
The CP-EBUS not only reaches the mediastinal lymph nodes outside the lumen, but also probes the blood vessels, including the aortic arch, left and right pulmonary artery (PA) trunk, azygos vein, hilum, and lobar arteries. The quality of US imaging of lesions at these sites obtained through the body surface is poor, with enhanced CT or angiography often needed for confirmation. Because these examination methods require injection of a contrast agent, use in patients with allergies, renal impairment, and pregnancy are limited. Therefore, many researchers attempt to diagnose pulmonary vascular disease, including PE and non-embolic pulmonary vascular disease (details below), using EBUS.
PE is a common medical condition. Currently, the main diagnostic methods for PE include enhanced chest CT and pulmonary ventilation perfusion scans. Intravenous drug administration is required for both methods, while 24% of the patients with suspected acute PE in the PIOPED II study have one or more of the contraindications to the use of contrast agents (30). Because the PA is very close to the central airway, it can be clearly examined using EBUS. In a prospective, multicenter pilot study (31), 32 patients, all of whom had central PA emboli with stable hemodynamics, were subjected to EBUS examinations within 24 hours following diagnosis of a PE by enhanced CT. The typical US characteristics of PEs are intravascular echo clumps obstructing the entire lumen or attached to the tube wall, with the embolus more easily identified by Doppler flow imaging techniques. The study results showed that the enhanced CT located 101 PEs, among which EBUS identified 97 (96%), with the four missed thrombi located in the PA of the middle or lower lobe. At least one PE was identified for each patient (100%). Such results confirmed the feasibility of using EBUS for the diagnosis of PEs.
The above research described the US imaging characteristics of PEs, suggesting that PEs should be considered suspicious if an echogenic mass is observed, which also provides more PE diagnostic options for patients not eligible for contrast injection. Because it was not a blind study design, the finding of the EBUS bronchoscopist would be affected by the CT pulmonary angiography results, leading to a higher rate of detecting PEs. To further prove the sensitivity of EBUS in the diagnosis of PEs, the researchers should use a single blind method specific to the EBUS bronchoscopist, or conduct an EBUS examination before the enhanced CT scan is obtained.
Because lung cancer is associated with a high risk of PE, some researchers have suggested that when using EBUS-TBNA for mediastinal lymph node staging determination in patients with lung cancer, the operator should also conduct routine exploration of peripheral pulmonary arteries to enable the early detection of PEs (32), which clearly increases the requirements for EBUS bronchoscopist.
Using EBUS we can observe the movement of thrombi in blood vessels, and the real-time thrombus dissolution process during thrombolytic therapy in patients, which might facilitate new clinical research findings.
Due to the distal diameter of 6.3-6.9 mm (depending on the brand) (6), CP-EBUS can only probe PEs adjacent to the central airway, which is a major limitation. Combination use with a radial probe EBUS for bronchiole PEs may compensate for such a deficiency, and may even detect small clots missed by enhanced CT scan.
Non-thrombotic endovascular lesions (NELs)
NELs include PA sarcomas, metastatic pulmonary aneurysm thrombi, and septic pulmonary emboli (33). Previously, to obtain confirmation, a surgical procedure or cardiac catheterization was usually needed, which was difficult. The emergence of EBUS provides a new solution for this situation. EBUS-TBNA at the vascular lesion can obtain the diagnosis. Due to aspiration of blood vessels, the safety of such procedure is an issue. In early 2015, Al-Saffar et al. (34) conducted a systemic review with 12 cases reported in 11 papers, with different degrees of tumor invasion or intravascular echo clumps of PA observed by EBUS examination. Among the cases, nine were diagnosed by EBUS-TBNA, and two of the remaining three cases were diagnosed by right heart catheterization, and the other case was diagnosed by PET/CT combined with enhanced CT. The final diagnoses were as follows: sarcoma (n=6); lung cancer (n=2); thyroid cancer (n=1); renal cell cancer (n=1); melanoma (n=1); and PE (n=1). There were no complications during the aspiration procedure. In addition to the 12 cases described in this systematic review, there were three NELs diagnosed by EBUS-TBNA reported by different researchers (35-37), all of which had no complications.
Using EBUS, the operator found PAs between the lesion and airway. Thus, some researchers were more aggressive in their attempts (38,39), punctured through the PA with the aspiration needle, and successfully performed fine needle aspiration biopsies of lesions outside the PA, with no bleeding or other complications. However, according to Botana-Rial et al. (40), when performing aspiration of lymph node lesions, the operator accidentally stabbed the PA, with the formation of a local intramural hematoma and hemopneumomediastinum, which significantly improved 24 hours later, as observed on CT scan.
The biggest advantage of EBUS-TBNA is that technology can obtain pathologic specimens. Most of the above reported cases were misdiagnosed as PEs before the EBUS-TBNA performed. For patients with pathologic changes within the vessels, if the size of the lesion does not change or increase after systemic anticoagulant therapy, physicians should consider the possibility of vascular tumor diseases, which can be diagnosed by EBUS-TBNA.
According to the above report, no severe complications were observed after aspiration within the PA lesions, suggesting that aspiration of PAs by biopsy needles is relatively safe. Some researchers, however, have pointed out that if patients also have chronic obstruction of a PA caused by a sarcoma or thromboembolism, then PA aspiration may lead to serious bleeding complications, in which case aspiration is not recommended (34). It is believed that the occurrence of complications is closely associated with the type of fine needle, puncture angle, degree of invasion of the PA by the lesions, and the PA pressure, while the safety evaluation regarding this procedure needs confirmation by animal and clinical experiments with large sample sizes.
Other applications for diagnosis
Thyroid nodule aspiration can be performed with EBUS guidance, which provides a new method for some difficult-to-diagnose patients with non-palpable intrathoracic thyroid nodules. In 2014, Kumar et al. summarized the results of eight patients who underwent EBUS-guided needle aspiration thyroid biopsies between 2006 and 2013 (41). Among these eight patients, there were four patients with papillary thyroid cancer, two patients with colloid thyroid adenomas, and two patients with benign diseases. In 2014 Casal et al. (42) reviewed 12 patients in their institution, which is by far the largest sample size of EBUS-guided thyroid nodule biopsies. All of these patients had successful aspirations with sufficient samples and no complications; three patients were diagnosed with malignant diseases (metastatic adenocarcinoma of the breast, large B-cell lymphoma, and metastatic adenocarcinoma of the lung), and the remaining nine patients had thyroid follicular nodules (8 of 9) and multinodular goiter (1 of 9). In contrast, Steinfort et al. (43) and Kennedy et al. (44) reported two patients with infectious complications after aspiration (spontaneous purulent discharge from the skin and a thyroid abscess); full recovery was achieved with antimicrobial treatment. As demonstrated in the above clinical studies and case reports, EBUS can detect thyroid disease with needle aspiration biopsy, and achieve high diagnostic capability, but with a relatively high infectious risk. Before aspiration, the bronchoscopist should take full advantage of the pros and cons. Due to the limited number of cases available, studies with larger sample sizes are needed for more accurate conclusions. Also, there is no research studying the evaluation of thyroid nodules using the elastographic features of EBUS, which warrants further study.
The trachea-bronchus is the most commonly infringed region by esophageal and thyroid cancers, and sufficient pre-operative assessment is critical for the selection of the optimal operative method and treatment regimen. EBUS can facilitate effective pre-operative evaluation of esophageal and thyroid cancers. It has been reported that the sensitivity and specificity of using EBUS in determining invasion of the trachea and bronchus by tumors of the adjacent organs were significantly higher than CT and magnetic resonance imaging (92% and 83%, respectively) (45). With a pre-operative evaluation by EBUS, unnecessary surgery could be spared for some patients (17 of 25). Such a study preliminarily showed the advantages of EBUS in pre-operative assessment. If combined with elastographic imaging (29), evaluation of tumor infiltration by EBUS may be more robust.
Due to the proximity of the heart to the central airway, EBUS has also been used to diagnose heart disease, including left atrial myxomas and puncturing pericardial effusion (46,47). Ashinuma et al. (48) reported a case of pericardial mesothelioma by EBUS-TBNA.
Moreover, even if there is a specified distance (≤30 mm) between the lesion and the central airway, aspiration can be implemented (49).
CP-EBUS for therapy
Drainage
Mediastinal cysts are one of the most common bronchopulmonary congenital malformations occurring in adults. Some mediastinal cysts may compress and irritate adjacent structures, thus causing symptoms. Nakajima et al. (50) reported one case of central airway stenosis caused by a mediastinal cyst, which was the first time that EBUS had been used for the treatment of mediastinal cysts, and the first time that EBUS had been used for therapeutic, but not diagnostic purposes. A CT scan showed a large mass compressing the trachea and narrowing the airway. EBUS-TBNA was performed, while the cyst was shown to be multilocular, with multiple aspirations required to drain the cyst. After that, the narrowed airway immediately recovered and the dyspnea resolved. No recurrence was observed during the 1-year follow-up. Casal et al. (51) reported a case of an infected mediastinal bronchogenic cyst successfully treated by EBUS-guided aspiration. A CT scan showed a well-circumscribed, round, 4.5-cm diameter lesion in the right lower paratracheal area. Two EBUS-guided aspirations were performed to drain the purulence within the cyst. Bacterial culture and drug susceptibility test results of the aspirate provided a reliable basis for the selection of effective antibacterial drugs. The patient was successfully cured by the above treatment.
A lymphangioma is a rare benign tumor that presents as a multicystic or sponge-like lesion formed by well-differentiated lymphatic tissue. Less than 1% of all lymphangiomas appear only in the mediastinum. Choi et al. (52) reported the first case of a successfully-treated mediastinal lymphangioma using EBUS (52). The patient was only 29 years of age and the tumor reached 13.7 cm in diameter. After the first EBUS aspiration, the diagnosis of lymphangiomas was confirmed, and within 2 days up to 700 mL of liquid was drained from the lesion. The patient was followed for 1 year post-operatively, with no disease recurrence or any complications observed.
For patients with mediastinal cysts or abscesses, if an EBUS-guided fine aspiration biopsy needle can be inserted into the tissue cysts, the cyst liquid can be drained for treatment, and surgery can be avoided. Because the treatment is minimally invasive and the procedure is relatively safe, even if the cyst recurs, aspiration drainage can still be repeated; however, it should be noted that if the cyst is malignant, surgical eradication should be considered first. For patients who cannot be cured or are not candidates for surgery, EBUS-guided needle aspiration should be used as palliative treatment.
Transbronchial needle injection (TBNI)
Endobronchial ultrasound guided-transbronchial needle injection (EBUS-TBNI) or dosing is another treatment method for mediastinal diseases; however, such reports are limited. One report involved EBUS-TBNI to deliver intra-tumoral therapy with cisplatin as adjuvant therapy to systemic chemotherapy in six patients with stage IIIa–IV non-small cell lung cancer (NSCLC) (53). This is the first study reporting anti-tumor drug injection of mediastinal lymph nodes through EBUS, with no serious complications observed. Khan et al. (54), for the first time, administered local drug injection into the recurrent tumor of patients with NSCLC; EBUS-guided direct injection of cisplatin into the tumor body was performed three times with no complications observed.
Mediastinum is a completely enclosed narrow space, which, once infected, is difficult to treat. The biggest concern of intra-mediastinal lesion drug injection should be the potential risk of infection. How to minimize the risk, thereby exploring new methods for the treatment of mediastinal tumors, will be the focus of a corollary study involving EBUS technology. In addition, for mediastinal tumor treatment, EBUS can be used not only for the local injection of chemotherapeutic drugs, but also the local implantation of radioactive particles, leading to effective control of the tumor. In contrast, local injection based on fiducial marker placement can accurately locate the central lung tumor for radical radiation treatment, rather than peripheral types only (55,56).
Conclusions
Ultrasonic bronchoscopy cannot only examine mediastinal lesions and guide aspiration, but also probe the blood vessels and thyroid around the airway, and may even be used to treat mediastinal diseases. For the bronchoscopist, EBUS examination is no longer limited to the observation of mediastinal lymph nodes (57), but also exploration of tissues outside the central airway, requiring not only skilled surgical ability, but also familiar knowledge of mediastinal anatomy, which raises unprecedented high requirements for the bronchoscopist, but is also necessary due to the development of medicine. For the development of EBUS technology, we expect the application of ultrasonic probe with higher resolution and smaller sizes, with other new technologies, such as three-dimensional US, ultrasonic contrast, and harmonic imaging, incorporated to further enhance the diagnostic ability of EBUS. At the same time, support from basic experimental research is also needed. Anayama et al. (58) have successfully created a rabbit VX2 lung tumor model for interventional studies. Therefore, further basic and clinical research can be carried out based on the current available study results to better utilize EBUS technology in clinical practice. In summary, EBUS technology has a broad prospect in application, which is based on the active development and efforts of medical professionals.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hürter T, Hanrath P. Endobronchial sonography: feasibility and preliminary results. Thorax 1992;47:565-7. [PubMed]
- Yasufuku K, Chhajed PN, Sekine Y, et al. Endobronchial ultrasound using a new convex probe: a preliminary study on surgically resected specimens. Oncol Rep 2004;11:293-6. [PubMed]
- Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8. [PubMed]
- Gompelmann D, Eberhardt R, Herth FJ. Endobronchial ultrasound. Endosc Ultrasound 2012;1:69-74. [PubMed]
- Schuhmann M, Eberhardt R, Herth FJ. Endobronchial ultrasound for peripheral lesions: a review. Endosc Ultrasound 2013;2:3-6. [PubMed]
- Figueiredo VR, Jacomelli M, Rodrigues AJ, et al. Current status and clinical applicability of endobronchial ultrasound-guided transbronchial needle aspiration. J Bras Pneumol 2013;39:226-37. [PubMed]
- Harris K, Maroun R, Attwood K, et al. Comparison of cytologic accuracy of endobronchial ultrasound transbronchial needle aspiration using needle suction versus no suction. Endosc Ultrasound 2015;4:115-9. [PubMed]
- Ozgul MA, Cetinkaya E, Kirkil G, et al. Lymph node characteristics of sarcoidosis with endobronchial ultrasound. Endosc Ultrasound 2014;3:232-7. [PubMed]
- Nakajima T, Yasufuku K, Yoshino I. Current status and perspective of EBUS-TBNA. Gen Thorac Cardiovasc Surg 2013;61:390-6. [PubMed]
- Santis G, Angell R, Nickless G, et al. Screening for EGFR and KRAS mutations in endobronchial ultrasound derived transbronchial needle aspirates in non-small cell lung cancer using COLD-PCR. PLoS One 2011;6:e25191. [PubMed]
- Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest 2013;143:1044-53. [PubMed]
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015;47:545-59. [PubMed]
- Um SW, Kim HK, Jung SH, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J Thorac Oncol 2015;10:331-7. [PubMed]
- Colella S, Vilmann P, Konge L, et al. Endoscopic ultrasound in the diagnosis and staging of lung cancer. Endosc Ultrasound 2014;3:205-12. [PubMed]
- Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006;130:710-8. [PubMed]
- Herth FJ, Eberhardt R, Krasnik M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest 2008;133:887-91. [PubMed]
- Fujiwara T, Yasufuku K, Nakajima T, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest 2010;138:641-7. [PubMed]
- Nakajima T, Anayama T, Shingyoji M, et al. Vascular image patterns of lymph nodes for the prediction of metastatic disease during EBUS-TBNA for mediastinal staging of lung cancer. J Thorac Oncol 2012;7:1009-14. [PubMed]
- Garra BS, Cespedes EI, Ophir J, et al. Elastography of breast lesions: initial clinical results. Radiology 1997;202:79-86. [PubMed]
- Lyshchik A, Higashi T, Asato R, et al. Thyroid gland tumor diagnosis at US elastography. Radiology 2005;237:202-11. [PubMed]
- König K, Scheipers U, Pesavento A, et al. Initial experiences with real-time elastography guided biopsies of the prostate. J Urol 2005;174:115-7. [PubMed]
- Foucher J, Chanteloup E, Vergniol J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006;55:403-8. [PubMed]
- Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005;128:343-50. [PubMed]
- Săftoiu A, Vilmann P, Gorunescu F, et al. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: a multicenter study. Endoscopy 2011;43:596-603. [PubMed]
- Popescu A, Săftoiu A. Can elastography replace fine needle aspiration? Endosc Ultrasound 2014;3:109-17. [PubMed]
- Figueiredo FA, da Silva PM, Monges G, et al. Yield of Contrast-Enhanced Power Doppler Endoscopic Ultrasonography and Strain Ratio Obtained by EUS-Elastography in the Diagnosis of Focal Pancreatic Solid Lesions. Endosc Ultrasound 2012;1:143-9. [PubMed]
- Trosini-Désert V, F, Taillade L, et al. Bronchial endoscopic ultrasound elastography: preliminary feasibility data. Eur Respir J 2013;41:477-9. [PubMed]
- Izumo T, Sasada S, Chavez C, et al. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol 2014;44:956-62. [PubMed]
- Inage T, Nakajima T, Yoshida S, et al. Endobronchial elastography in the evaluation of esophageal invasion. J Thorac Cardiovasc Surg 2015;149:576-7. [PubMed]
- Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317-27. [PubMed]
- Aumiller J, Herth FJ, Krasnik M, et al. Endobronchial ultrasound for detecting central pulmonary emboli: a pilot study. Respiration 2009;77:298-302. [PubMed]
- Sanz-Santos J, Andreo F, García-Olivé I, et al. Diagnosis of acute pulmonary embolism by endobronchial ultrasound as an incidental finding. Respiration 2011;81:150-1. [PubMed]
- Ye R, Zhao L, Wang C, et al. Clinical characteristics of septic pulmonary embolism in adults: a systematic review. Respir Med 2014;108:1-8. [PubMed]
- Al-Saffar F, Ibrahim S, Seeram V, et al. Use of endobronchial ultrasound to evaluate nonthrombotic endovascular lesions in pulmonary arteries: a systematic review. J Bronchology Interv Pulmonol 2015;22:28-32. [PubMed]
- Kamaleshwaran KK, Pattabiraman V, Mehta S, et al. Spindle cell sarcoma of pulmonary artery mimicking thromboembolism with lung metastasis detected in fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography. Indian J Nucl Med 2014;29:249-51. [PubMed]
- Modi K, Dhillon S, Kumar A, et al. Leiomyosarcoma of the pulmonary artery diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Endosc Ultrasound 2014;3:249-51. [PubMed]
- Caraway NP, Salina D, Deavers MT, et al. Pulmonary artery intimal sarcoma diagnosed using endobronchial ultrasound-guided transbronchial needle aspiration. Cytojournal 2015;12:3. [PubMed]
- Vincent B, Huggins JT, Doelken P, et al. Successful real-time endobronchial ultrasound-guided transbronchial needle aspiration of a hilar lung mass obtained by traversing the pulmonary artery. J Thorac Oncol 2006;1:362-4. [PubMed]
- Boujaoude Z, Pratter M, Abouzgheib W. Transpulmonary artery needle aspiration of hilar masses with endobronchial ultrasound: a necessary evil. J Bronchology Interv Pulmonol 2013;20:349-51. [PubMed]
- Botana-Rial M, Núñez-Delgado M, Pallarés-Sanmartín A, et al. Intramural hematoma of the pulmonary artery and hemopneumomediastinum after endobronchial ultrasound-guided transbronchial needle aspiration. Respiration 2012;83:353-6. [PubMed]
- Kumar A, Mohan A, Dhillon SS, et al. Substernal thyroid biopsy using Endobronchial Ultrasound-guided Transbronchial Needle Aspiration. J Vis Exp 2014.e51867. [PubMed]
- Casal RF, Phan MN, Keshava K, et al. The use of endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of thyroid lesions. BMC Endocr Disord 2014;14:88. [PubMed]
- Steinfort DP, Johnson DF, Irving LB. Infective complications from endobronchial ultrasound-transbronchial needle aspiration. Eur Respir J 2009;34:524-5. [PubMed]
- Kennedy MP, Breen M, O'Regan K, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of thyroid nodules: pushing the boundary too far? Chest 2012;142:1690-1. [PubMed]
- Wakamatsu T, Tsushima K, Yasuo M, et al. Usefulness of preoperative endobronchial ultrasound for airway invasion around the trachea: esophageal cancer and thyroid cancer. Respiration 2006;73:651-7. [PubMed]
- Cetinkaya E, Yılmaz A, Özgül A, et al. Left atrial mass demonstrated during endobronchial ultrasound session. Respiration 2011;81:57-8. [PubMed]
- He MC. Dr. Paul Zarogoulidis: the exploration on pneumothorax and new use of EBUS. J Thorac Dis 2015;7:S73-4. [PubMed]
- Ashinuma H, Shingyoji M, Yoshida Y, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in a patient with pericardial mesothelioma. Intern Med 2015;54:43-8. [PubMed]
- Dincer HE, Gliksberg EP, Andrade RS. Endoscopic ultrasound and/or endobronchial ultrasound-guided needle biopsy of central intraparenchymal lung lesions not adjacent to airways or esophagus. Endosc Ultrasound 2015;4:40-3. [PubMed]
- Nakajima T, Yasufuku K, Shibuya K, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the treatment of central airway stenosis caused by a mediastinal cyst. Eur J Cardiothorac Surg 2007;32:538-40. [PubMed]
- Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg 2010;90:e52-3. [PubMed]
- Choi SH, Kim L, Lee KH, et al. Mediastinal lymphangioma treated using endobronchial ultrasound-guided transbronchial needle aspiration. Respiration 2012;84:518-21. [PubMed]
- Hohenforst-Schmidt W, Zarogoulidis P, Darwiche K, et al. Intratumoral chemotherapy for lung cancer: re-challenge current targeted therapies. Drug Des Devel Ther 2013;7:571-83. [PubMed]
- Khan F, Anker CJ, Garrison G, et al. Endobronchial ultrasound-guided transbronchial needle injection for local control of recurrent non-small cell lung cancer. Ann Am Thorac Soc 2015;12:101-4. [PubMed]
- Harley DP, Krimsky WS, Sarkar S, et al. Fiducial marker placement using endobronchial ultrasound and navigational bronchoscopy for stereotactic radiosurgery: an alternative strategy. Ann Thorac Surg 2010;89:368-73; discussion 373-4. [PubMed]
- Steinfort DP, Siva S, Kron T, et al. Multimodality guidance for accurate bronchoscopic insertion of fiducial markers. J Thorac Oncol 2015;10:324-30. [PubMed]
- Konge L, Colella S, Vilmann P, et al. How to learn and to perform endoscopic ultrasound and endobronchial ultrasound for lung cancer staging: A structured guide and review. Endosc Ultrasound 2015;4:4-9. [PubMed]
- Anayama T, Nakajima T, Dunne M, et al. A novel minimally invasive technique to create a rabbit VX2 lung tumor model for nano-sized image contrast and interventional studies. PLoS One 2013;8:e67355. [PubMed]


