
Anatomical location and number of metastatic lymph nodes for prognosis of non-small cell lung cancer
Introduction
Lymph node metastasis is one of the most significant predictors of prognosis in patients with non-small cell lung cancer (NSCLC) (1). The 8th edition of the tumor, node, and metastasis (TNM) classification of lung cancer has been the staging standard worldwide since January 2017. The T and M descriptors were subdivided, whereas there were no major changes in the N descriptor (2). The N descriptor in lung cancer is currently determined solely by the anatomical locations of metastatic lymph nodes (mLNs), which have been identified using large-scale data analysis (1). Therefore, even the latest edition of TNM staging has failed to provide for the variability due to heterogeneity of the patient population. Several studies have demonstrated the usefulness of applying the number of mLNs (1,3-6), lymph node stations (7,8), lymph node zones (8,9), or lymph node metastatic ratios (10-12) to the nodal classification system to predict prognosis in patients with NSCLC. Multiple researchers have reported on the heterogeneity of prognosis at the same nodal stage, and the current N descriptor may have room for improvement (3,4,6,10,11,13). In other carcinomas, such as colorectal, gastric, and breast cancer, the number of mLNs is considered in the N descriptor (14).
It is unclear whether the number of mLNs affects the prognosis of patients with NSCLC. Therefore, we conducted a retrospective study to answer this question using a multicenter database. We hypothesized that the prognosis would be poorer as the number of mLNs increased. The aim of this study was to investigate the correlation between the number of mLNs and prognosis in patients with NSCLC who had undergone complete resection.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-390).
Methods
Patients
Using a multicenter database (Kanagawa Cancer Center, Hiroshima University, and Tokyo Medical University), we retrospectively reviewed the medical records of 2,662 patients who underwent R0 resection for lung cancer between 2010 and 2016. Complete resection is defined as no residual tumor, either macroscopically or microscopically, at each institution. R0 resection includes complete resection and uncertain resection, which means that a dissection of three mediastinal and hilar-intrapulmonary nodal stations, so that the final specimen includes at least six lymph nodes, are not met. In our study, 69 cases met the above conditions (15). We excluded patients who underwent wedge resection/segmentectomy (n=711), had limited node dissection (n=273), were diagnosed with unclassifiable or small cell lung carcinoma (n=28), had an unknown number or station for lymph node metastasis (n=79), or died within 30 days after surgery (n=4). Limited node dissection means ND0-1 without mediastinal lymph node dissection. In our study, the minimum number of lymph nodal stations is 6. However, the minimum number of lymph nodes is two, since the dissection area contains only fat and may not include lymph nodes. This study does not include patients with neoadjuvant chemotherapy. The tumor stage was determined according to the 7th edition of the Union for International Cancer Control Tumor, Node, Metastasis classification for malignant tumors (16). All resected lymph nodes were classified based on the nodal map defined by the International Association for the Study of Lung Cancer (IASLC) (9). We collected clinicopathological variables, which included age, sex, smoking history, carcinoembryonic antigen (CEA), clinical stage, surgical procedure, lymphadenectomy, resected lymph nodes, metastatic lymph nodes, histologic type, pathological stage, pleural invasion, pulmonary metastasis, lymphatic vessel invasion, blood vessel invasion, EGFR mutation, adjuvant therapy, and prognosis. All cases were cT1a–4N0–2M0. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of 24-18 and waived the requirement for informed consent from individual patients.
Imaging and lymph node dissection
Computed tomography (CT) images of the chest were acquired using a 16-row, multi-slice CT scanner with or without enhancement. The tumor size was estimated using high-resolution CT (HRCT) scans with a section thickness of 1–2.5 mm. Lymph nodes were defined as positive for metastasis if they had a short axis diameter ≥10 mm as measured by HRCT or if the uptake of fluorine-18 fluorodeoxyglucose was greater than that of surrounding normal structures using positron emission tomography (PET) imaging. We performed PET imaging in all patients and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) or mediastinoscopy if necessary to minimise the risk of undetected N2 disease. We performed systematic lymph node dissection for clinical N1 or N2 patients and lobe-specific lymph nodal dissection for clinical N0 patients. Systematic lymph node dissection was defined as dissection of the hilar lymph nodes and all ipsilateral mediastinal lymph nodes located at stations 2R, 4R, 7, 8, and 9 for cancers found on the right side and stations 4L, 5, 6, 7, 8, and 9 for cancers on the left side (17). Lobe-specific lymph node dissection was defined as dissection of hilar lymph nodes and specific mediastinal lymph nodes depending on the location of the primary lung cancer (stations 2R and 4R for the right upper lobe, stations 4L, 5, and 6 for the left upper lobe, and stations 7, 8, and 9 for the lower lobe of each side).
Follow-up
All patients were followed up once every 3 months for the first 2 years and every 6 months thereafter. The follow-up examinations included a physical assessment, chest radiography, chest CT, and blood tests for the tumor marker carcinoembryonic antigen. PET and brain magnetic resonance imaging were performed in cases where the patient complained of neurological symptoms.
Statistical analysis
The date of recurrence was defined as the date that disease recurred and was confirmed by imaging. Confirmation of recurrence were determined either radiologically or histologically. Recurrence-free survival (RFS) was defined as the period beginning with surgery to the date of recurrence or the last known recurrence-free date. Overall survival (OS) was defined as the period beginning with surgery to the date of death (regardless of disease) or survival confirmation. Survival curves were plotted using the Kaplan–Meier method, and differences between groups were determined using the log-rank test. Multivariate analyses were performed using the Cox proportional hazards model. In the multivariable analyses, we included the covariate variables of age, sex, smoking history, histologic type, pathological T descriptor, and mLNs after checking for explanatory variables that are closely related to each other. Statistical analyses were conducted using SPSS software, version 23 (IBM, Armonk, NY, USA). All P values were two-sided, and values <0.05 were considered statistically significant.
Results
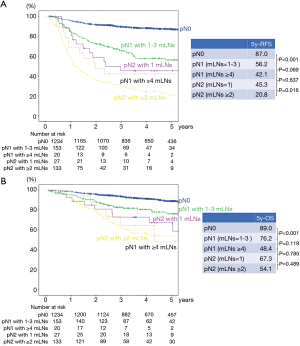
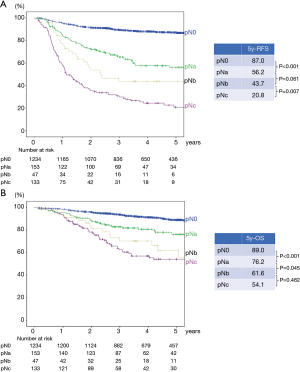
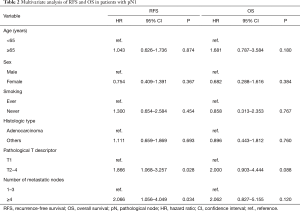
A total of 1,567 patients were analyzed in our study (Figure 1). Table 1 presents the clinicopathological characteristics of the patients. The median follow-up period was 49 months. We performed adjuvant chemotherapy in 500 patients (31.9%). There were 326 recurrences, of which local recurrences were 168 cases and distant metastases were 145 cases (13 cases were unknown). The recurrence pattern of pN1–2 included 85 local recurrences and 88 distant metastases. The clinical N stages cN0, cN1, and cN2 were present in 84.0%, 12.0%, and 4.0% of the patients, respectively. The pathological N stages pN0, pN1, and pN2 were present in 78.8%, 11.0%, and 10.2% of the patients, respectively. Regarding pN2, upstaging of 6% from cN2 was observed. We performed systematic lymph node dissection in 16.7% and lobe-specific lymph node dissection in 83.3% of the patients. The median number of resected lymph nodes was 16, and the median number of metastatic lymph nodes in patients with pN1–2 disease was 2. Regarding pN1, lymph node metastasis to hilar (#10), interlobar (#11), lobar (#12), segmental (#13), and subsegmental (#14) was observed in 16 (9.2%), 44 (25.4%), 74 (42.8%), 26 (15.0%), 4 cases (2.3%), respectively (5 cases were unknown). There were significant differences in the 5-year RFS and OS rates between the pN0, pN1, and pN2 groups (the 5-year RFS rates for pN0, pN1, and pN2 were 87.0%, 54.5%, and 24.7%, respectively, P<0.001 and P<0.001 for pN0 vs pN1 and pN1 vs pN2, respectively; the 5-year OS rates for pN0, pN1, and pN2 were 89.0%, 73.8%, and 56.7%, respectively, P<0.001 and P=0.001 for pN0 vs pN1 and pN1 vs pN2, respectively.) The RFS and OS curves overlapped each other for pN1 patients with 1, 2, and 3 mLNs and survival was greater for these groups than for patients with 4–6 mLNs (Figure S1A,B). Consequently, we analyzed patients with ≤3 mLNs and ≥4 mLNs separately in the pN1 group. As shown in Figure 2, pN1 patients with ≥4 mLNs (n=20) tended to have a worse prognosis than those with ≤3 mLNs (n=153); however, we could not show a statistically significant difference in RFS and OS. In patients with pN2 disease, those with 1 mLN had a relatively good prognosis, and there were no differences between patients with ≥2 mLNs. Accordingly, we analyzed patients with 1 mLN and ≥2 mLNs separately in the pN2 group (Figure S1C,D). Patients in the pN2 disease group with ≥2 mLNs (n=133) tended to have a worse prognosis than those with 1 mLN (n=27), and RFS was statistically significant. The numbers of patients with ≥4 mLNs in the pN1 group and 1 mLN in the pN2 group were small, but their RFS curves almost overlapped each other. Therefore, pN1 (mLNs ≥4) and pN2 (mLNs =1) were analyzed as one group. In Figure 3, pNa, pNb, and pNc represent the pN1 group with 1–3 mLNs, pN1 group with ≥4 mLNs plus pN2 group with 1 mLNs, and pN2 group with ≥2 mLNs, respectively. The pNb group had a worse prognosis than the pNa group, and a better prognosis than pNc group (Figure 3). Multivariate analysis showed that the pathological T descriptor and number of mLNs were independently associated with recurrence in patients with pN1 disease (Table 2). In patients with pN2 disease, only the number of metastatic nodes was independently associated with recurrence (Table 3).
Full table
Full table
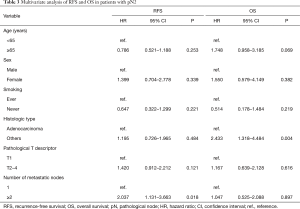
Full table
Discussion
This study investigated the correlation between the number of mLNs and prognosis in patients with NSCLC who underwent complete resection. We demonstrated that patients with 4 or more mLNs in pN1 disease and 2 or more mLNs in pN2 disease had a worse prognosis. By combining the anatomical location with the number of mLNs and dividing them into 3 groups, we found differences in survival rates between the groups. Our studies are the first to propose a new nodal stage that combines pN1 with ≥4 mLNs and pN2 with 1 mLN. Our data show that subdividing the nodal classification system based on a combination of anatomical location and the number of metastatic lymph nodes may predict the prognosis of NSCLC.
In the current TNM classification, the N descriptor is still defined only by the anatomical location; however, this definition has some unsatisfactory aspects. The heterogeneity of prognosis at the same nodal stage is a problem that needs to be solved. Therefore, subdivision of the nodal stage has been proposed (1,3-10). In the lung cancer staging project of the IASLC, Asamura et al. concluded that the N descriptor in the 7th edition of the TNM classification accurately predicted prognosis, suggesting that the number and station of mLNs may affect prognosis (1). Our data is identical with the IASLC-proposed N staging system for pN2, but not for pN1. The point is that the anatomical location of mLNs is considered to be the most important factor in the nodal staging of lung cancer. Therefore, future subdivisions of the N descriptor will need to be combined with the current classification of anatomical locations to improve prognosis. Saji et al. reported that in patients with pN1 and pN2 disease, those with a number of mLNs ≥4 had a worse prognosis than patients with 1–3 mLNs, and their results regarding pN1 were identical to ours (3). Some previous studies reported that patients with a number of mLNs ≥2 had a worse prognosis than patients with 1 mLNs in those with pN1 and pN2 disease (1,6). In our study, 4 or more mLNs in pN1 and 2 or more mLNs in pN2 were the cutoff value for each N descriptor. This may suggest that patients with pN1 and pN2 disease have different characteristics of lymph node metastasis. In other words, the extent of lymph node metastasis may be more important in patients with pN2 than pN1. The reason is that the pN2 group with 1 mLN suggest skip N2 metastasis without N1 involvement, which indicates a good prognosis (18,19). The pN2 (mLNs =1) group is a different population in patients with pN2 disease, and the number of mLNs may be less important in the pN2 (mLNs ≥2) group. The fact that the number of mLNs did not affect the prognosis in pN2 (mLNs ≥2) group means that the anatomical location of mLNs (in other words, metastasis to the N2 region) is more important than the number of mLNs in pN2 (mLNs ≥2) group. We propose a new nodal stage that combines pN1 with ≥4 mLNs and pN2 with 1 mLN. Our study provides information that supports the specific subdivision of the N descriptor in the upcoming revision of the TNM staging system.
Multivariate analysis showed that the number of mLNs was independently associated with recurrence, but not with survival, in patients with pN1 and pN2 disease. In addition, we could not observe a statistically significant difference in OS between pNb (pN1 group with ≥4 mLNs plus pN2 group with 1 mLNs) and pNc (pN2 group with ≥2 mLNs) (Figure 3). One of the reasons is that the number of cases in the pN1 (mLNs ≥4) and pN2 (mLNs =1) groups was small. OS may be affected by treatment after recurrence. We should validate whether our new nodal stage shows a statistically significant difference in prognosis at each T stage, which requires a large number of patients. We believe that future large-scale studies could resolve this. In addition, multivariate analysis in Table 3 showed that patients with adenocarcinoma had a better prognosis than those with non-adenocarcinoma. The reason is that patients with adenocarcinoma have the opportunity to use new drugs such as tyrosine kinase inhibitor when they relapsed. In addition, patients with squamous cell carcinoma may be associated with interstitial pneumonia, and it may be difficult to receive effective treatment at the time of recurrence.
In clinical practice, patients with lymph node metastases will receive adjuvant chemotherapy. Therefore, it is difficult to compare pN0 with pN1 and pN2 without adjuvant therapy. If the number of lymph node metastases correlates with prognosis, we might need to change the management of the patients. For patients with a poor prognosis, we may consider performing more intensive chemotherapy or shortening the follow-up interval after surgery.
We acknowledge that our study has several limitations. First, because our study was retrospective, a validation cohort study will be required to support our results. Second, the numbers of lymph nodes were counted differently depending on whether the nodes were removed en bloc or separately (fragmented lymph nodes) (20). Lymphadenectomy methods were different for dissection or sampling according to the surgeon performing the work. Lymph nodes sometimes become one group, and the boundaries of individual nodes may not be recognized. Therefore, it may be difficult to accurately determine the number of mLNs. Third, systematic lymph node dissection was only performed for clinical N1 or N2 patients. In our study, the small number of systematic lymph node dissection may cause bias. The clinical trial Z0030 of the American College of Surgeons Oncology Group showed no survival difference between patients who underwent systematic lymph node dissection and mediastinal lymph node sampling for N0 or N1 NSCLC (21). There is also no unified recommendation for lymph node sampling between the guidelines (17,22). The NCCN guideline recommend one or more nodes sampling from all mediastinal stations. It is unclear if there is no significant difference in prognosis between systematic and lobe-specific lymph nodal dissection. We conducted this study assuming that the prognosis of systematic and lobe-specific lymph nodal dissection is similar for patients with cN0. Fourth, the tumor stage was categorized according to the 7th edition of TNM classification, not the 8th edition in this study. There are no major changes in the N descriptor between the 7th and 8th edition, therefore we consider that the results of the 7th edition can be extrapolated to the 8th edition. Fifth, it is unknown whether our data can be applied to non-surgical cases, since our study only included patients who underwent surgical resection. It is difficult to recognize the number of mLNs using preoperative images. Therefore, it is unknown whether pathological stages can be applied to clinical nodal stages in lung cancer. Further investigation will be necessary using other datasets, including non-surgical patients.
In conclusion, our results suggest that nodal classification that combines subdivisions of the number of metastatic lymph nodes with their anatomical location may predict prognosis of NSCLC. Although American Joint Committee on Cancer (AJCC) recommended future subclassification of nodal stage that includes the number of lymph nodes (23), our study provides one concept to set nodal classifications in the upcoming revision of the TNM staging system. If the number of mLNs predicts prognosis, it is necessary to accurately diagnose the number of mLNs or to puncture multiple lymph nodes via EBUS or mediastinoscopy before surgery. This will help identify groups with poor prognosis and actively treat them. To clarify its prognostic impact, we need prospective large-scale data with a uniform patient background.
Acknowledgments
We would like to thank Editage (
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-390
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-390
Peer Review File: Available at https://dx.doi.org/10.21037/jtd-21-390
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-390). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of 24-18 and waived the requirement for informed consent from individual patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Asamura H, Chansky K, Crowley J, et al. International Association for the Study of Lung Cancer, A.B.M. Prognostic Factors Committee, I. Participating, The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Goldstraw P, Chansky K, Crowley J, et al. International Association for the Study of Lung Cancer, A.B. Prognostic Factors Committee, I. Participating, S. International Association for the Study of Lung Cancer, B. Prognostic Factors Committee Advisory, I. Participating, The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Saji H, Tsuboi M, Shimada Y, et al. A proposal for combination of total number and anatomical location of involved lymph nodes for nodal classification in non-small cell lung cancer. Chest 2013;143:1618-25. [Crossref] [PubMed]
- Wei S, Asamura H, Kawachi R, et al. Which is the better prognostic factor for resected non-small cell lung cancer: the number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol 2011;6:310-8. [Crossref] [PubMed]
- Lee JG, Lee CY, Park IK, et al. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg 2008;85:211-5. [Crossref] [PubMed]
- Katsumata S, Aokage K, Ishii G, et al. Prognostic Impact of the Number of Metastatic Lymph Nodes on the Eighth Edition of the TNM Classification of NSCLC. J Thorac Oncol 2019;14:1408-18.
- Kang CH, Ra YJ, Kim YT, et al. The impact of multiple metastatic nodal stations on survival in patients with resectable N1 and N2 nonsmall-cell lung cancer. Ann Thorac Surg 2008;86:1092-7. [Crossref] [PubMed]
- Yun JK, Lee GD, Choi S, et al. Comparison between lymph node station- and zone-based classification for the future revision of node descriptors proposed by the International Association for the Study of Lung Cancer in surgically resected patients with non-small-cell lung cancer. Eur J Cardiothorac Surg 2019;56:849-57. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Jonnalagadda S, Arcinega J, Smith C, et al. Validation of the lymph node ratio as a prognostic factor in patients with N1 nonsmall cell lung cancer. Cancer 2011;117:4724-31. [Crossref] [PubMed]
- Ding X, Hui Z, Dai H, et al. A Proposal for Combination of Lymph Node Ratio and Anatomic Location of Involved Lymph Nodes for Nodal Classification in Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1565-73. [Crossref] [PubMed]
- Chiappetta M, Leuzzi G, Sperduti I, et al. Lymph-node ratio predicts survival among the different stages of non-small-cell lung cancer: a multicentre analysis. Eur J Cardiothorac Surg 2019;55:405-12. [Crossref] [PubMed]
- Caldarella A, Crocetti E, Comin CE, et al. Prognostic variability among nonsmall cell lung cancer patients with pathologic N1 lymph node involvement. Epidemiological figures with strong clinical implications. Cancer 2006;107:793-98. [Crossref] [PubMed]
- Union for International Cancer Control (UICC). In: Brierley JD, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 8th ed. Hoboken, NJ: Wiley-Blackwell; 2017.
- Rami-Porta R, Wittekind C, Goldstraw P. Complete Resection in Lung Cancer Surgery: From Definition to Validation and Beyond. J Thorac Oncol 2020;15:1815-8. [Crossref] [PubMed]
- Union for International Cancer Control (UICC) In: Sobin LH, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours. 7th ed. Hoboken, NJ: Wiley-Blackwell; 2009.
- Lardinois D, Leyn PD, Schil PV, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Wang L, Ye G, Xue L, et al. Skip N2 Metastasis in Pulmonary Adenocarcinoma: Good Prognosis Similar to N1 Disease. Clin Lung Cancer 2020;21:e423-34. [Crossref] [PubMed]
- Tsitsias T, Okiror L, Veres L, et al. New N1/N2 classification and lobe specific lymphatic drainage: Impact on survival in patients with non-small cell lung cancer treated with surgery. Lung Cancer 2021;151:84-90. [Crossref] [PubMed]
- Zhong WZ, Liu SY, Wu YL. Numbers or Stations: From Systematic Sampling to Individualized Lymph Node Dissection in Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:1143-45. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer (Version 4.2021). 2021. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- American Joint Committee on Cancer (AJCC) In: Amin MB, Edge S, Greene F, eds. AJCC Cancer Staging Manual. Springer; 2017.



