
The new allocation era and policy
Introduction
The lung allocation system for lung transplantation has undergone two major changes in policy over the last 20 years. The first major change was the implementation of the lung allocation score (LAS) replacing waiting time as the primary determinant for donor lung allocation. LAS is a composite score derived from a variety of objective medical criteria that are used to calculate the probability of one-year waitlist survival and one-year post-transplant survival. The implementation of the LAS had significant effects on lung transplant candidates, recipients and transplant centers (1). Overall, the LAS facilitated allocation based on medical urgency and post-transplant outcomes, and initially it substantially improved waitlist mortality and allocation efficiency (1). Unfortunately, the LAS based allocation did not address the geographic variability in donor lung availability and inequity in access. Prior to November 24, 2017, lungs were allocated first within the local donation service area (DSA) managed by the local organ procurement organization (OPO). Donor lungs were allocated to the ABO blood group matched/compatible candidate with the highest LAS within the local DSA. If local transplant center(s) did not accept the donor lung(s), they would then be offered to candidates at transplant centers in increasing concentric circles from the donor hospital of 500 nautical miles (termed zones A through E). However, after a lawsuit against the United States Department of Health and Human Services (DHHS), the primary geographic allocation unit was changed from the local DSA to 250 nautical mile circle surrounding the donor hospital (2).
The current geographic allocation system is only temporary as the Organ Procurement and Transplantation Network (OPTN) and the United Network for Organ Sharing (UNOS) are redesigning organ allocation under a new continuous distribution framework that can be tailored to each organ as needed (3). We will review the history of donor lung allocation, the effects of the major changes in the system and describe how lung allocation may change in the next few years.
Early history of lung allocation
The first official donor lung allocation system in the U.S. was started by UNOS in 1990, and lungs were distributed first to patients within their local DSA based primarily on waiting time and ABO blood group compatibility (4,5). Due to the observation of high waitlist mortality associated with idiopathic pulmonary fibrosis (IPF), a relatively small modification was made to the lung allocation policy in 1995, and candidates with IPF were granted a 90-day credit when placed on the waiting list (4,5).
Although this was a step in the right direction, it did not solve the problem of increasing waitlist time and mortality. Since waiting time was the primary determinant of lung transplantation, transplant physicians increasingly placed patients on the waiting list long prior to needing a transplant to allow them to accrue time and increase their chances of receiving a transplant when they needed it (4). As a result, the waiting list size and waiting list mortality steadily increased (1,4). In 1998, the DHHS issued the Final Rule as a directive to the OPTN to address the inequities in organ allocation (6).
The “Final Rule” directed that organ allocation be based primarily on medical urgency using objective measurable criteria to the extent possible (6). The Institute of Medicine (IOM) issued an independent assessment of organ allocation policies and the Final Rule (7,8). The IOM concluded that waiting time should not be used as allocation criterion to the extent possible, organs should be shared broadly, and post-transplant survival and outcomes should be considered in distribution to avoid futile transplants (7,8). Additionally, the IOM recommended continual oversight to monitor allocation processes and performance measures for transplant centers, OPOs and the OPTN (7).
In response to the Final Rule, the OPTN thoracic organ transplantation committee formed the thoracic organ allocation modelling subcommittee (also known as the lung allocation subcommittee) to address the directives from the Final Rule (4,9). After several years of intense work, debate and planning, the OPTN and UNOS responded with the first dramatic change to lung allocation in May 2005 by implementation of the LAS as the primary determinant for lung allocation (1). The LAS uses an algorithm that attempts to estimate transplant benefit by calculating a waitlist urgency measure and a post-transplant survival measure. The waitlist urgency measure is based on calculation of expected days lived during an additional year on the waiting list whereas the post-transplant survival measure is based on calculation of expected days lived during first year post-transplant (Table 1) (10). The waitlist urgency measure is weighted twice while the post-transplant survival measure is weighted only once for calculation of transplant benefit measure and raw allocation score. The raw allocation score is then normalized so that LAS ranges between 0 and 100 (10).
Table 1
| Waitlist urgency measure | Post transplant survival measure |
|---|---|
| Age at Offer | Age at Offer |
| Bilirubin mg/dL | Cardiac index (L/min/m2) |
| Bilirubin increase of at least 50% | Continuous mechanical ventilation |
| Body Mass Index (BMI) (kg/m2) | Creatinine (serum) mg/dL |
| Cardiac Index L/min/m2 | Creatinine increase ≥150% |
| Central Venous Pressure (mmHg) | Diagnosis |
| Continuous mechanical ventilation | Group A: Obstructive Lung Disease |
| Creatinine (serum) mg/dL | Group B: Pulmonary Vascular Diseases |
| Diagnosis | Group C: Cystic Fibrosis |
| Group A: Obstructive Lung Disease | Group D: Restrictive Lung Diseases |
| Group B: Pulmonary Vascular Disease | Functional status |
| Group C: Cystic Fibrosis | Oxygen need at rest (L/min) |
| Group D: Restrictive Lung Disease | Six-minute walk distance (feet) |
| Functional status | |
| Forced Vital capacity (FVC) % predicted pCO2 | |
| pCO2 increase of at least 15% | |
| Oxygen need at rest (L/min) | |
| Six-minute walk distance (feet) | |
| Pulmonary Artery (PA) systolic pressure at rest |
LAS, lung allocation score.
Calculation of the LAS:
Raw Allocation Score = Post transplant survival measure – 2 × (waitlist urgency measure)
Post-transplant survival measure = Expected days lived during 1st year after transplant
Waitlist urgency measure = Expected days lived during additional year on the waiting list
The raw allocation score ranges from ‒730 to 365 and is then normalized to a LAS of 0–100. Normalization leads to the following corresponding LAS: A raw score of ‒730 = LAS of 0 and a raw score of 365 = LAS of 100 (10).
Additionally, Lung Review Boards were created to allow an avenue for physicians to appeal for higher exception scores if they believed that their patients were sicker than what their LAS reflected (4,11). In such cases when a physician believes that a patient’s calculated LAS underestimates that patient’s potential transplant benefit, one can submit a narrative with appropriate medical justification (4,11).
Effects of the LAS
There were several positive observed effects after the implementation of the LAS based allocation system. First the number of transplants rose at a more rapid rate after launching of the LAS, rising from an average increase of 45 per year from 2000 to 2004 to 91 per year from 2006 to 2011 (1). Secondly and perhaps more importantly, the waiting list mortality decreased by 40% from an average of 500 to 300 annually (12). After the LAS was implemented, the waiting list size immediately decreased substantially because there no longer was a benefit to having a patient on the waiting list to accrue time (8). This likely contributed to an increase in allocation efficiency thereby increasing transplant volume and decreasing waitlist mortality (8).
Perhaps unsurprisingly, the characteristics of the typical transplant candidate and recipient changed over time as well. The transplant candidate and recipient population became much older, with an increasing proportion of transplants occurring in persons over 65 years of age and IPF emerged as the predominant indications for transplant (5,13). Most importantly, the transplant recipients have become sicker at the time of transplant with a steady rise in LAS since 2005 (13). In recent years, more candidates have been hospitalized in the intensive care unit prior to transplant and the proportion of patients on mechanical support, in particular ECMO prior to transplant has risen as well (13).
Changes to lung allocation since LAS implementation in 2005
Although the LAS offers an objective measure of urgency taking into account post-transplant survival, it is by no means perfect. A numeric value cannot accurately capture a specific candidate’s risk of death or post-transplant survival, and the LAS certainly had a number of limitations that UNOS and OPTN have attempted to address.
The OPTN board of directors approved a major revision of the LAS in 2012 that was formally implemented on February 19th 2015 (14,15). These changes included addition of serum creatinine, change in PaCO2, total bilirubin and cardiac index into the LAS calculation (15). These changes had the largest impact on patients with PAH and the new score estimated pre-transplant mortality more accurately in PAH patients (8,16).
Unfortunately, the LAS was validated in a cohort of patients aged 12 and older, and therefore adult and adolescent lungs allocated using the LAS could not be allocated to children less than 12. The consequences of this system came to head when the family of Sarah Murnaghan, a critically ill 10-year-old girl awaiting lung transplantation for cystic fibrosis, appealed for an exception to this policy in 2013 which was denied. Ms. Murnaghan’s family then appealed to the media and politicians, and eventually a federal judge granted Ms. Murnaghan access to adult donors (17). The OPTN/UNOS quickly approved the Adolescent Classification Exception for Pediatric Candidates, which allowed candidates less than 12 years old to apply for an exception through the Lung Review Board in order to access adolescent and adult donor lungs allocated with LAS. Additionally, a more comprehensive review was conducted and a new policy that permitted exception for candidates less than 12 years old, allowed broader sharing for candidates less than 18 years old and allowed candidates less than 12 years old to receive a deceased donor lung of any compatible blood type was implemented on 3/30/2017 (18).
Change to geographic allocation in November 2017
Lung allocation based on LAS has largely been an indisputable success, and it is certainly consistent with the Final Rule mandate. However, despite the Final Rule mandate to distribute organs over broad geographic areas, the LAS based system did not address geographic variability in lung availability and patient access. Prior to November 24, 2017, the primary geographic unit for lung allocation was the local DSA. Lungs were offered to candidates outside the local DSA in 500 nautical mile increments only if there was no local transplant center or if the local transplant center(s) declined the lungs.
DSAs are very heterogeneous in size, population and the number of available donor organs (19). As a result of DSA-based lung allocation system and following implementation of the LAS, donor lungs were frequently allocated to lower priority candidates within a local DSA when there were multiple higher priority candidates nearby (20,21). The DSA based system resulted in dramatically different outcomes for candidates on the waiting list based on where they were listed (19).
The second major change to lung allocation occurred on November 24, 2017 after a waitlisted candidate in New York city filed a lawsuit with the DHHS to broaden the geographic sharing of donor lungs (2). The DHHS directed the OPTN and its contractor UNOS to emergently review the lung allocation policy and within five days of the lawsuit, the lung allocation system was modified dramatically. The new primary lung allocation unit became a 250 nautical mile radius surrounding the donor hospital rather than the DSA. OPTN and UNOS selected a 250 nautical mile distance instead of a longer distance for the primary allocation unit due to concerns with increasing ischemic time, increasing cost and decreasing efficiency in lung allocation (2).
Effects of the geographic allocation change
After 250 nautical mile radius was adopted as the primary allocation unit on an emergency basis by the OPTN/UNOS executive committee, the new policy proposal was opened for public comment (22). The OPTN executive committee, the thoracic organ transplantation committee and the transplant community at large identified several concerns with the proposed policy. These concerns included longer cold ischemic time, arbitrary proposed distance, increased travel distance to recover organs, increased travel cost, unknown long-term impact on post-transplant outcomes, unknown impact to low volume/small center programs and impact on specific diagnoses groups (22). The committee acknowledged all the relevant concerns but approved the emergent policy change on an interim basis and requested a two-year extension to allow a review of alternative allocation systems (22).
Using the Thoracic Simulated Allocation Model, a simulation model developed by Scientific Registry of Transplant Recipients (SRTR), Mooney et al., simulated the effect of changing the primary allocation unit from the local DSA to 500 nautical miles and beyond. These investigators demonstrated that broader sharing would result in a 21.3% reduction in waitlist mortality by increasing the primary allocation unit to 500 nautical miles and 31.8% reduction in waitlist mortality by increasing the allocation unit to 1,000 nautical miles (23). This reduction was primarily observed in Group D (interstitial lung disease diagnoses) candidates and those with LAS above 50. Finally, they also reported slightly lower 30-day and 1-year post-transplant survival with broader organ sharing (24).
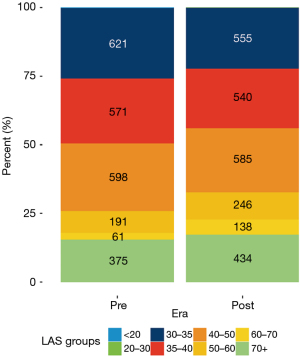
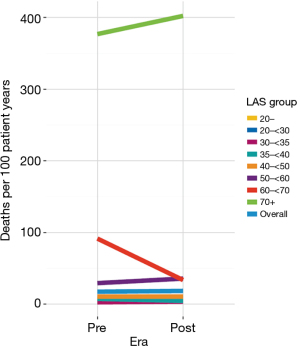
Due to the sudden nature of the change, OPTN/UNOS has monitored the allocation change closely and OPTN/UNOS published a one year report in January 2019 (22,25). Given the broader geographic sharing, there were several predictable changes to the waitlist and transplant recipient population. After the allocation change, the match LAS at transplant increased from 47.25 in the pre geographic allocation change era to 49.61 in the post geographic allocation change era (25). The number of transplants for recipients with a LAS above 50, particularly for recipients with LAS at transplant above 60 increased notably in the post era (Figure 1) (25). Overall, there was not a statistically significant difference in the death rate for waitlist candidates between the pre and post geographic allocation change eras, although there was a statistically significant decrease in the death rate for candidates in the LAS group 60–70 (Figure 2) (25). There was not a statistically significant change in waiting list death rate based on diagnosis or blood type or in most other LAS subgroups (25).
The ischemic time and the average distance between the transplant center and donor hospital increased after the geographic allocation change (25). This has resulted in a decrease in the number of local lungs allocated to a transplant center within the local DSA and more lungs allocated regionally and nationally (Figure 3) (25).

Nationally, donor lung utilization rate was not statistically different in the post era compared to the pre era (25). However, the sequence number of the final accepting candidate for donor lungs is higher in the post era compared to the pre era, and the time from first electronic offer to cross clamp has increased (25). This may represent increase in cost, effort and time for OPOs organizing and coordinating transplants.
The future of lung allocation
The geographic allocation change potentiated the trend of rising LAS at the time of transplant, which was not unexpected. Unfortunately, high LAS patients have the most post-transplant morbidity and potentially lower one-year post-transplant survival (24,26-29). A decrease in post-transplant survival is undoubtedly concerning; however, the medical management of candidates with high LAS has improved considerably over the last decade (30). Additionally, another concern with an increasing allocation distance is the longer ischemic time, increased costs in lung procurement, and decreased allocation efficiency for OPOs and transplant centers (31).
To examine the geographic distribution of organs and establish guiding principles for the use of geographic limits in organ allocation, an Ad Hoc Geography Committee was formed in December 2017 (32). The OPTN/UNOS board of directors approved the following principles of Geographic Distribution on June 12, 2018 (32).
Geographic distribution may be constrained in order to
- Reduce inherent differences in the ratio of donor supply and demand across the country;
- Reduce travel time expected to have clinically significant effect on ischemic time and organ quality;
- Increase organ utilization and prevent organ wastage;
- Increase efficiencies of donation and transplant system resources (32).
After deliberation and analysis, the Ad hoc Geography Committee identified three organ allocation frameworks that could be applied to all organ allocation policies and proposed them for public comment in August 2018 (3). These frameworks were (I) Fixed distance from the donor hospital, (II) mathematically optimized boundaries, and (III) continuous distribution (3,32).
Organ distribution based on fixed distance from the donor hospital would be similar to the current geographic allocation system that was implemented in November 2017 (3,32). The main challenge in this system is how to select the optimal fixed distance to balance travel time costs, efficient organ allocation, ischemic time/organ quality, and medical urgency/priority. A fixed distance too far would overemphasize medical urgency/priority whereas a short fixed distance would likely overemphasize travel costs and ischemic time/organ quality. A mathematical optimization model for organ distribution could use several factors to estimate an optimal organ distribution distance/area including pre-transplant deaths, organ supply, organ demand and/or travel time (3,32). This optimization model can allow for broader organ sharing based on organ supply and/or population density while also placing constraints to account for efficient and cost effective OPO/transplant center operation (3,32). Finally, a continuous distribution framework without geographic boundaries incorporates the proximity of candidates to a donor and the medical urgency/priority to create a composite allocation score (3,32). This model would eliminate the fixed borders separating donors and waitlist candidates, and all organ transplant systems could utilize the same framework. It could be further tailored to account for specific clinical characteristics and ischemic considerations of each organ (3,32).
These three proposals were put forth for public comment and voted on by OPTN Regions at the regional meetings. A continuous distribution algorithm was clearly the preferred distribution model, and in December 2018 the OPTN board of directors approved the continuous distribution framework for all allocation systems (32).
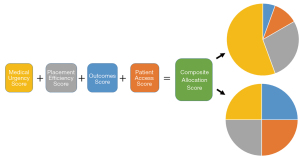
In August 2019, OPTN Thoracic Organ Transplantation Committee published a concept paper for continuous distribution of donor lungs that proposed creating a composite allocation score based on medical urgency, efficiency, outcomes and patient access (33). The medical urgency score reflects priority based on objective medical criteria regarding risk of death on the waiting list. The efficiency score would estimate the ability to allocate organs quickly. The outcomes score would estimate post-transplant benefit from a transplant similar to post-transplant component of the LAS. In addition, the patient access score would promote access to transplant for patient populations at greater risk of longer waiting time and higher mortality, such as pediatric patients, multi-organ transplant candidates and highly sensitized candidates. As the committee considers the components of the composite allocation score, they will have to determine how to weigh each component (Figure 4) (33).
A sample transplant match-run best illustrates the benefits of a continuous distribution system and pitfalls of a rigid geographic border based system. Consider two candidates with similar LAS (Table 2). Candidate 1 is very far from the donor hospital but has a minimally higher LAS while candidate 2 is very close the donor hospital. In a rigid geographic boundary based system, such as the current lung allocation system, donor lung(s) would be allocated to candidate 1. However, there is likely no clinically meaningful difference in the medical urgency of these candidates, but there are considerable differences in organ allocation efficiency. A continuous distribution system would allow for the allocation to the local candidate due to its efficiency score component (33).
Table 2
| Candidate 1 | Candidate 2 | |
|---|---|---|
| LAS | 50.1 | 50 |
| Distance to Donor Hospital | 249 nautical miles | 5 nautical miles |
LAS, lung allocation score.
Now consider a second scenario with two other candidates (Table 3); candidate 1 has a much higher LAS than candidate 2, but is located slightly further than candidate 2 from the donor hospital. Candidate 1 is just further than 250 nautical miles falling outside the rigid geographic boundaries in the current lung allocation system. Although the allocation of lung(s) to candidate 1 would be similarly efficient, rigid geographic boundaries preclude allocation of lungs to the candidate who has the much higher LAS and medical urgency (33).
Table 3
| Candidate 1 | Candidate 2 | |
|---|---|---|
| LAS | 90 | 45 |
| Distance to Donor Hospital | 252 nautical miles | 249 nautical miles |
LAS, lung allocation score.
A continuous lung distribution system could effectively address the limitations in the current rigid geographic boundary based system and allow the allocation of lungs to local candidates when there are not clinically meaningful differences in medical urgency and outcomes. Furthermore, a continuous distribution system allows for more variables to be introduced into the algorithm that are in line with the mandates of the Final Rule (33). The OPTN Thoracic Organ Transplantation Committee in conjunction with the Board of Directors created a continuous distribution workgroup to construct the new allocation system (32,33). This process will include the identification of attributes for inclusion in the composite allocation score, categorization and prioritization of attributes, building a framework, SRTR modeling followed by public comment and finally board approval (33).
The committee has proposed and asked for feedback on possible attributes for inclusion into a points-based composite allocation score (33). In the concept paper, the working group has proposed the following attributes: medical urgency, age, blood type, waiting time, sensitization, and proximity (Figure 5). As attributes are assessed for inclusion into the composite allocation score, the committee will be charged with ensuring that each attribute connects to the goals of the OPTN Final Rule. The committee will have to determine how attributes should be prioritized, compared and weighted appropriately (33). Prioritizing and weighting of potential attributes against each other is possibly the most arduous task when generating the new composite allocation score. How can we effectively compare medical urgency and utility well? The committee has acknowledged this challenge and will attempt to achieve this through transparent partnership with the transplant community (33).
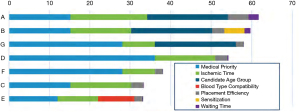
The OPTN Thoracic Organ Transplantation Committee is using feedback from public comment to help guide the formation of the composite allocation score, and the continuous distribution working group is actively working to build a points-based system prioritizing and weighting the various attributes. After creation of the initial models for organ allocation, the SRTR will model the frameworks under consideration prior to educating and soliciting further feedback from the transplant community. This process will likely take several years (33).
Conclusions
Lung allocation has undergone two major changes in the last two decades, first with the implementation of LAS as the primary determinant of donor lung allocation in 2005 and second, the change in the primary geographic allocation unit from local DSA to a 250-nautical mile radius in 2017. Both of these modifications helped improve the allocation system and make it more consistent with the Final Rule. The LAS system helped reduce waitlist mortality and improve the efficiency of donor lung allocation. However, the LAS system did not address the geographic differences that exist in donor availability and hence disparities in patient access to lungs. The change of the primary allocation unit DSA to 250 nautical mile radius is more consistent with the Final Rule and helped mitigate the effects of geographic variability in donor lung supply. However, rigid geographic boundaries continue to leave certain patients at a disadvantage without sound medical or utility reasons.
A continuous distribution system is expected to positively impact the allocation of donor lungs. It will permit several attributes other than the LAS to contribute to a points-based system that generates a composite allocation score consisting of variables such as medical urgency, post-transplant outcomes, travel distance, waiting time, organ allocation efficiency, and patient access to donor organs (based on blood group, sensitization, pediatric candidates and size). The major challenge ahead for the OPTN/UNOS, the Thoracic Organ Transplantation Committee and the lung transplant community at large is to ensure that the selected attributes are weighted appropriately. Overall, a continuous distribution framework for donor lung allocation has the potential to improve the current lung allocation system further and be more consistent with the Final Rule.
Acknowledgments
We thank all the members of our Lung Transplant Program for their tireless work and daily sacrifice to care for our patients with advanced lung disease before and after lung transplantation. We also would like to acknowledge the generosity of the Boomer Esiason Foundation for supporting our patient care and research programs.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Jonathan D’Cunha) for the series “Lung Transplantation: Past, Present, and Future” published in Journal of Thoracic Disease. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-2021-17). The series “Lung Transplantation: Past, Present, and Future” was commissioned by the editorial office without any funding or sponsorship. Both authors have no other conflicts of interest to declare.
Ethical Statement: Both authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant 2016;35:433-9. [Crossref] [PubMed]
- Callahan L, Uccellini K. Broader Sharing of Adult Donor Lungs. OPTN/UNOS Executive Committee. Accessed 1/31/2020. Available online: https://optn.transplant.hrsa.gov/media/2314/broader_sharing_lungs_20171124.pdf
- Prentice M. Public Comments Proposal: Frameworks for Organ Distribution. UNOS, Dec 2018. Accessed 1/31/2020. Available online: https://optn.transplant.hrsa.gov/media/2565/geography_publiccomment_201808.pdf
- Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant 2006;6:1212-27. [Crossref] [PubMed]
- Pierson RN 3rd, Barr ML, McCullough KP, et al. Thoracic organ transplantation. Am J Transplant 2004;4:93-105. [Crossref] [PubMed]
- Organ Procurement and Transplantation Network. Health Resources and Services Administration, HHS. Final rule. Fed Regist 1999;64:56650-61. [PubMed]
- Institute of Medicine. Organ Procurement and Transplantation: Assessing Current Policies and the Potential Impact of the DHHS Final Rule. Washington, DC: National Academy Press, 1999.
- Egan TM. How Should Lungs Be Allocated for Transplant? Semin Respir Crit Care Med 2018;39:126-37. [Crossref] [PubMed]
- Organ Procurement and Transplantation Network Thoracic Organ Transplant Comittee Chaired by Cliff H Van Meter Jr. Report of the Thoracic Organ Transplantation Committee to the UNOS Board of Directors. Baltimore, MD: November 18-19, 1999.
- A Guide to Calculating the Lung Allocation Score. UNOS. Accessed 1/30/2020. Available online: https://unos.org/wp-content/uploads/unos/lung_allocation_score.pdf
- Lung Review Board Information. Accessed 1/30/2020. Available online: https://optn.transplant.hrsa.gov/media/2701/review_board_guidelines_lung.pdf
- George TJ, Arnaoutakis GJ, Beaty CA, et al. Acute kidney injury increases mortality after lung transplantation. Ann Thorac Surg 2012;94:185-92. [Crossref] [PubMed]
- Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Lung. Am J Transplant 2019;19:404-84. [Crossref] [PubMed]
- OPTN Lung Allocation System (LAS) Changes: Frequently Asked Questions. UNOS. Accessed 1/31/2020. Available online: https://optn.transplant.hrsa.gov/media/1157/las_qa.pdf
- Changes to the lung allocation system. UNOS. Accessed 1/31/2020. Available online: https://optn.transplant.hrsa.gov/news/changes-to-the-lung-allocation-system/
- Pasupneti S, Dhillon G, Mooney J. Lung Transplant Wait list Outcomes After Implementation of the Revised Lung Allocation Score. J Heart Lung Transplant 2018;37:S183. [Crossref]
- Ladin K, Hanto DW. Rationing lung transplants--procedural fairness in allocation and appeals. N Engl J Med 2013;369:599-601. [Crossref] [PubMed]
- Modify pediatric lung lung policy. UNOS. Accessed 1/30/2020. Available online: https://optn.transplant.hrsa.gov/governance/public-comment/modify-pediatric-lung-policy/
- Benvenuto LJ, Anderson DR, Kim HP, et al. Geographic disparities in donor lung supply and lung transplant waitlist outcomes: A cohort study. Am J Transplant 2018;18:1471-80. [Crossref] [PubMed]
- Iribarne A, Meltzer DO, Chauhan D, et al. Distribution of donor lungs in the United States: a case for broader geographic sharing. Clin Transplant 2016;30:688-93. [Crossref] [PubMed]
- Russo MJ, Meltzer D, Merlo A, et al. Local allocation of lung donors results in transplanting lungs in lower priority transplant recipients. Ann Thorac Surg 2013;95:1231-4; discussion 1234-5. [Crossref] [PubMed]
- Callahan L, Uccellini K. Modifications to the Distribution of the Deceased Donor Lungs. OPTN/UNOS Thoracic Organ Transplantation Committee. Accessed 1/31/2020. Available online: https://optn.transplant.hrsa.gov/media/2523/thoracic_boardreport_201806_lung.pdf
- Mooney JJ, Bhattacharya J, Dhillon GS. Effect of broader geographic sharing of donor lungs on lung transplant waitlist outcomes. J Heart Lung Transplant 2019;38:136-44. [Crossref] [PubMed]
- Maxwell BG, Mooney JJ, Lee PH, et al. Increased resource use in lung transplant admissions in the lung allocation score era. Am J Respir Crit Care Med 2015;191:302-8. [Crossref] [PubMed]
- Lehman R, Carrico B. Monitoring of the Lung Allocation Change, 1 Year Report: Removal of DSA as a Unit of Allocation. UNOS, 16 Jan 2019. Accessed 1/31/2020. Available online: https://optn.transplant.hrsa.gov/media/2815/20190116_thoracic_committee_report_lung.pdf
- Hayanga JA, Lira A, Vlahu T, et al. Lung Transplantation in Patients with High Lung Allocation Scores in the US: Evidence for the Need to Evaluate Score Specific Outcomes. J Transplant 2015;2015:836751 [Crossref] [PubMed]
- Braun AT, Dasenbrook EC, Shah AS, et al. Impact of lung allocation score on survival in cystic fibrosis lung transplant recipients. J Heart Lung Transplant 2015;34:1436-41. [Crossref] [PubMed]
- Horai T, Shigemura N, Gries C, et al. Lung transplantation for patients with high lung allocation score: single-center experience. Ann Thorac Surg 2012;93:1592-7; discussion 1597. [Crossref] [PubMed]
- Hayanga JWA, Shigemura N, Aboagye JK, et al. ECMO Support in Lung Transplantation: A Contemporary Analysis of Hospital Charges in the United States. Ann Thorac Surg 2017;104:1033-9. [Crossref] [PubMed]
- Crawford TC, Grimm JC, Magruder JT, et al. Lung Transplant Mortality Is Improving in Recipients With a Lung Allocation Score in the Upper Quartile. Ann Thorac Surg 2017;103:1607-13. [Crossref] [PubMed]
- Snyder JJ, Salkowski N, Wey A, et al. Organ distribution without geographic boundaries: A possible framework for organ allocation. Am J Transplant 2018;18:2635-40. [Crossref] [PubMed]
- Castro, S. Briefing Paper: Frameworks for Organ Distribution. UNOS, Dec 2018. Accessed 1/31/2020. Available online: https://optn.transplant.hrsa.gov/media/2762/geography_boardreport_201812.pdf
- Alcorn, J. Concept Paper: Continuous Distribution of Lungs. UNOS, August 2019. Accessed 1/31/2020. Available online: https://optn.transplant.hrsa.gov/media/3111/thoracic_publiccomment_201908.pdf


