
International expert opinion on the use of nebulization for pediatric asthma therapy during the COVID-19 pandemic
Introduction
Asthma treatment and management guidelines are being updated in response to the current coronavirus disease 2019 (COVID-19) pandemic. While the updated guidelines broadly agree in their recommendations of asthma medication, guidance on the use of nebulizers for the delivery of asthma medication is contradictory (1-5). Nebulizers generate aerosol particles 1–5 μm in size, which can carry viruses into the lungs (6). The risk of infection transmission via droplet nuclei and aerosols may increase during nebulization owing to the potential generation of high volumes of respiratory aerosols that may be propelled over a longer distance than in natural dispersion patterns (7,8). Additionally, large aerosolized particles may stimulate a cough reflex in both patients and bystanders and thus increase transmission risk (7,9). Based on these potential risks, concerns have been raised regarding the possibility that nebulizer use in patients with COVID-19 infection could transmit potentially viable infection to susceptible bystander hosts. However, existing data are limited and of poor quality (10) and so it remains unclear whether nebulization poses a transmission risk for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Guidelines differ on how to address this uncertainty. Some, including those from Global Initiatives for Asthma (GINA), the Australian Commission on Safety, and Quality in Healthcare and the Canadian Thoracic Society recommend avoiding the use of nebulizers in asthma treatment where possible (1-3,5). By contrast, recent guidelines from the National Institute for Health and Care Excellence (NICE), UK and the New and Emerging Respiratory Viral Threat Assessment Group (NERVTAG) advise that the use of nebulizers can be continued since the aerosol generated during nebulization comes from a non-patient source (the fluid in the nebulizer chamber) and therefore does not contain virus particles derived from the patient (11,12). Position statements from the French Language Respiratory Society (SPLF) and the Haute Autorité de Santé (HAS) support the continued use of all asthma medications provided relevant safety measures for healthcare providers (HCPs) and relatives are maintained during nebulization (4). The contradictory guidance from the above societies could leave treating physicians unable to provide clear advice on optimal therapy plans to their asthma patients. This poses a particular problem for pediatricians as nebulization is the only suitable treatment modality for some of their patients.
Guidelines, including those from GINA, recommend the use of pressurized metered dose inhalers (pMDIs) plus a spacer, as an alternative to nebulizers (1-3,13). However, asthma drug administration by nebulization has many medical benefits compared with administration by pMDI as it allows co-administration of other compatible medications, administration of high doses of medication and furthermore, oxygen could be used as the driving force for nebulization in patients with acute exacerbation and severe hypoxemia (14-18). Nebulization is also a more user-friendly method compared with drug delivery by pMDI as it requires minimal patient cooperation, does not require patients to hold their breath, permits easy dose adjustments and is straightforward to use (14,19,20). Moreover, nebulization results in the rehydration of airways through inhalation and may also improve patient compliance due to ease of use (21,22). Correct use of a pMDI requires patient cooperation and good hand-lung coordination, which is often difficult to achieve for patients such as children, elderly, and those with restricted hand dexterity due to comorbid conditions (16,20,22,23).
Inhaled therapy is the gold standard treatment strategy for pre-school children, i.e., aged 5 years and younger, as it delivers a high concentration of medication in the airways and results in a rapid onset of action with fewer systemic side effects compared with an oral delivery method (1,24). Inhaled corticosteroids (ICS) are commonly used for the treatment of acute and chronic airway inflammation (25). Nebulization therapy with ICS is widely used in children of all ages for acute and chronic asthma due to the aforementioned advantages (14,25). The choice of device should therefore be based on the child’s age and capability, and in this respect, nebulized corticosteroids are reported to be the most appropriate delivery method for pre-school children (26).
However, due to the publication of contradictory guidelines on the use of nebulizers since the start of the COVID-19 pandemic, there is a need for clear guidance on the safe use of nebulization, both in the hospital and home setting, especially in pediatric asthma, as this patient population is most dependent on this mode of drug delivery. This need was confirmed at an expert advisory group meeting held in December, 2020. The objective of the present work is therefore to provide evidence-based, clinically relevant, and practical recommendations on the use of nebulization in different settings during the COVID-19 pandemic to physicians and other HCPs involved in the treatment of pediatric asthma.
We present the following article in accordance with the AGREE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-841).
Methods
A literature review was conducted via PubMed searches using selected search terms to identify English language publications relevant to pediatric management of asthma using nebulizers during the COVID-19 pandemic (Table 1). Additional searches included relevant guideline publications for SARS-CoV-1 (Table 1). The search terms specified in Table 1 were chosen to identify articles published on the following topics: pandemics, the desired disease area, treatment options, and existing guidelines. Specific omissions were used to narrow the search results. For COVID-19-related and SARS-CoV-1-related searches, articles published from November, 2019 to February, 2021 and from January, 2003 to February, 2021 were retrieved, respectively. The search was restricted to full journal articles including narrative reviews, systematic reviews, meta-analysis studies, randomized clinical trials, original research and observational studies, case series, position statements and selected reviews addressing the covered questions or cross-references from these publications.
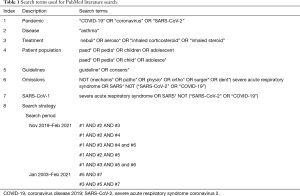
Full table
Search results were reviewed to remove duplicate articles, and then abstracts were reviewed and articles relevant to the topics discussed were selected for further analysis. Other selected articles included cross-references from PubMed search results and non-English language articles contributed by the authors. The results of the literature review were used to identify areas of consensus and areas that required further discussion.
Due to travel restrictions and social distancing requirements, a classic Nominal Group Technique (NGT) approach was not possible. As such, a modified NGT, as described by McMillan et al. [2016], was used to generate consensus statements (27). As previously mentioned, the need to establish a consensus was identified during a virtual Advisory Board meeting hosted by the sponsor in December, 2020. The advisory panel consisted of 11 international experts. All panel members were subsequently invited to participate in the consensus process. Potential consensus statements were formulated based on the independent generation of ideas by the participating panel members. An electronic document containing potential statements was then individually reviewed by all panel members and responses were documented electronically. The ranking process of potential consensus statements was carried out during a second virtual meeting held in March, 2021 and led by an independent moderator. Following discussions, a questionnaire containing close-ended questions was prepared, based on areas of disagreement. To maintain confidentiality, the voting was performed by sending the questionnaire separately to each author, via email. Areas of disagreement were resolved by soliciting ‘Yes’ or ‘No’ votes from the panel in response to the close-ended questions. A consensus was defined as ≥70% ‘Yes’ votes and therefore mandated the inclusion of the respective consensus statement. Revisions and validations were obtained from panel members following two rounds of individual reviews of the consensus statements and final approval was obtained.
Results
A flowchart of the literature review conducted using pre-defined search terms (Table 1) is provided in Figure 1. Out of 152 ‘COVID-19’-related articles and 23 ‘SARS-CoV-1’-related articles, 26 publications were selected for further analysis. The literature search did not identify any guidelines that gave advice to pediatricians or primary care physicians on the use of nebulization in pediatric asthma treatment during the pandemic. Therefore, it was confirmed that a consensus paper on this topic based on the expertise of the advisory panel members could help address this gap in the literature. All 11 international experts who were invited to participate in the consensus process agreed to participation.
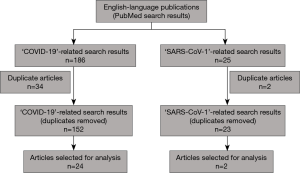
Four broad topics in the context of pediatric nebulization were identified:
- Nebulization is an aerosol generating procedure (AGP) that poses transmission risk of COVID-19:
- Consensus reached: 100% agreement;
- Nebulization should be continued during the pandemic:
- Consensus reached: 100% agreement;
- Need for guidance on risk mitigation in hospital and home settings:
- Consensus reached: 100% agreement;
- The need for guidance on nebulization in the school setting was a sub-category which achieved a consensus of 73% agreement;
- Need for further data on the risk of COVID-19 transmission:
- Consensus reached: 73% agreement.
Nebulization is an AGP which poses a transmission risk of COVID-19 if the patient is infected with COVID-19
Currently, there are concerns regarding the high transmissibility of SARS-CoV-2 while using AGPs. Evidence for potential infection transmission associated with various medical procedures primarily came from studies investigating the SARS-CoV-1 outbreak of 2002–2003 (28). Although nebulization is considered an AGP, previous studies investigating the risk of SARS-CoV-1 transmission from nebulization reported no significant increase in infection risk (28,29). A systematic review on AGPs and transmission risk published in 2012 concluded that there was no significantly elevated risk of SARS-CoV-1 transmission to healthcare staff from patients receiving nebulizer treatment (29).
Despite the lack of clear evidence, there is ongoing concern regarding the transmission risk associated with nebulization. Nebulization as a procedure with COVID-19 transmission risk was partially based on conclusions extrapolated from SARS-CoV-1 studies (28). It must be noted that it is not the nebulization procedure per se that poses a risk of transmission of COVID-19, provided the nebulization device has been disinfected or sterilized correctly, but the patient receiving nebulization therapy, if he/she is infected with COVID-19. If the recipient of nebulization therapy is infected with SARS-CoV-2, a transmission risk to those in the surrounding area cannot be ruled out, especially in the absence of adequate protective measures.
The need to continue nebulization in pediatric asthma care during the pandemic
There is a clear medical need for nebulization in pre-school children, in children who cannot operate a pMDI plus spacer, in children with impaired asthma control when using the pMDI plus spacer, in children with severe exacerbations, or children in respiratory distress. Expert reviews and recommendations on nebulization for asthma advocate its use in cases where patients show a poor response to a pMDI plus spacer, in children who are uncooperative or unable to follow the directions for pMDI use or in the case of medication shortages (9,19,22,23,30). There are also concerns of improper use of pMDI plus spacer following an abrupt switch from the patient’s usual method of drug delivery (31). Position statements including those from French respiratory societies and the Australian health agency also support the use of nebulization in those patient groups in which nebulization is the only optimal method of asthma drug delivery (4,32).
Nebulization is vital in aborting the development of exacerbations. In those patients with evident improvement following acute treatment in a healthcare setting, nebulization may have to be performed in home settings as a continuation of the acute treatment (33). Withholding nebulization therapy may also lead to respiratory failure in severe cases (34). There are concerns regarding the overabundance of caution that HCPs and health institutions might exercise in their approach to the use of nebulizers that may lead to unfavourable patient outcomes (35). Hence, there is a need to shift the focus to risk-benefit assessment when it comes to the use of nebulizers in pediatric asthma management.
Limiting the transmission risk of SARS-CoV-2 is a critical component of infection control during the pandemic. On this front, there have been reports on issues around hospital-acquired-SARS-CoV-2 infection in many parts of the world, especially in the absence of adequate safety measures (36,37). Discontinuing corticosteroid therapy, including nebulization, would put children at risk of exacerbations and could necessitate hospital and emergency room visits (25,38). Such hospital visits could potentially increase the risk of contracting COVID-19 for both patients and carers (25,38,39). Although concerns around hospital-acquired-SARS-CoV-2 and transmission risk of nebulization in a hospital setting could be potentially seen as a cause-and-effect dilemma, proper patient care should not be compromised. In fact, children with asthma constitute a patient population wherein age-specific challenges exist in diagnosis and optimal treatment (40,41). Therefore, HCPs responsible for pediatric asthma management must be extremely cautious about issues that could arise from any deviation from their patients’ effective usual therapy involving nebulization to avoid worsening patient outcomes.
Nevertheless, given the uncertainty around transmission risk of SARS-CoV-2 arising from nebulization of COVID-19 positive patients, adequate protective measures need to be in place to ensure the safety of patients, carers and HCPs. Therefore, it is imperative to understand and adhere to key safety measures while performing nebulization.
Recommendation 1: nebulization in pediatric asthma care during the pandemic should be continued but with caution and adequate protective measures in place.
Guidance on measures for risk mitigation when performing nebulization in various settings
Early in the pandemic, a study from two hospitals in Wuhan, China, reported an effective reduction of SARS-CoV-2 RNA concentration in aerosols following implementation of safety measures (42). Many guidelines now clearly indicate the effectiveness and importance of such safety measures for controlling the spread of infection (43,44). Regardless of whether a patient is COVID-19 positive or not, we recommend that the following standard safety precautions be adhered to in all nebulization settings.
Aseptic technique
Hand hygiene is considered one of the most effective tools to prevent the transmission risk of pathogens including SARS-CoV-2 (44,45). For observing strict hand hygiene, we recommend the use of soap (wash for at least 20 seconds and dry using disposable towels) or hand sanitizer (at least 70% alcohol), before and after treating patients (44).Moreover, proper aseptic technique must be used to avoid contamination of aerosol reservoirs and nebulizer medication (46,47). We recommend the use of gloves, face masks and long-sleeved disposable gowns while setting up the nebulizer and handling medication. This recommendation is also a precautionary measure to mitigate the risk of SARS-CoV-2 transmission to patients from HCPs or carers who might be asymptomatic (47).
In a designated area or room where nebulization is carried out, cleaning and disinfection of surrounding areas including high-touch surfaces is important to prevent contact transmission, especially in the case of suspected or confirmed COVID-19 (44). In alignment with the WHO guidelines, we recommend thorough cleaning and disinfection of worktops, desks, door handles, and other areas of the room once nebulization has been performed using 70% ethanol and a contact time of at least one minute.
Physical distancing
Close proximity, prolonged contact, high frequency of contacts and confined shared environments are high-risk factors for air-borne transmission (48). Therefore, HCPs and carers must keep a distance of at least 1.5 m while performing nebulization. In those cases where very young children need an adult nearby during the procedure, extreme care must be taken to adhere to the respective recommended safety measures for the different settings in which nebulization will be performed (Table 2).
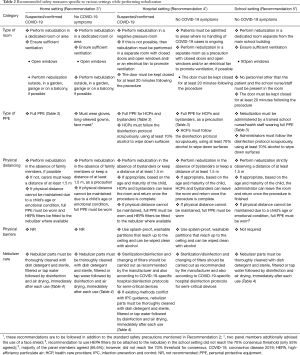
Full table
Recommendation 2: summary of standard safety precautions:
- Follow strict hand hygiene: use soap (wash for at least 20 seconds and dry using disposable towels) or hand sanitizer (at least 70% alcohol);
- Use aseptic technique while setting up the equipment and dispensing medication: use gloves, face masks and long-sleeved disposable gowns;
- Maintain physical distancing of at least 1.5 m;
- Thoroughly clean and disinfect general surfaces of the room where nebulization is performed: use 70% ethanol with a minimum contact time of 1 minute.
Safety measures specific to home settings
Cleaning and disinfection/sterilization of semi-critical medical devices such as nebulizers is of utmost importance in infection control as inadequate care of equipment poses a huge risk for infection transmission (49,50). It is important to clean nebulizer parts prior to disinfection or sterilization as inadequate cleaning will affect the effectiveness of these processes (52). The method of disinfection or sterilization depends on the setting in which these procedures are carried out and whether clear manufacturer’s instructions for the same are available. If clear manufacturer’s instructions on cleaning, disinfecting/sterilizing and storing are available, these should be strictly followed after each use. However, it must be noted that some of the instructions pertaining to disinfection might contradict prevailing infection prevention and control (IPC) guidelines (52,53). For example, for semi-critical devices, current guidelines from the Center for Disease Control (CDC) recommend decontamination and utilization of high-level disinfection after each use, which might be in conflict with the instructions of the user manual. Additionally, according to one report, several instruction manuals or package inserts of nebulizers recommend the use of vinegar for cleaning, which will render disinfection ineffective (53).
In the absence of an instruction manual on device care or guidance on regular disinfection pertaining to the current pandemic situation, we recommend cleaning nebulizer parts with dish detergent and sterile, filtered, or tap water followed by disinfection and air drying, immediately after each use (50,53). In the home setting, disinfection can be performed using either a cold or a heat method (Table 4).
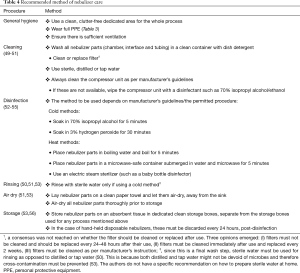
Full table
The transmission risk of SARS-CoV-2 indoors tends to be higher in poorly ventilated spaces compared with well-ventilated spaces (57). At home, especially if the patient has suspected or confirmed COVID-19, it is imperative to perform nebulization in a well-ventilated area. Ideally, family members should be absent during nebulization, although we recognize that this might not be possible if the child is of a very young age or appears distressed if left alone. In such situations, it is highly recommended that the nebulizer be fitted with high-efficiency particulate air (HEPA) filters and that full personal protective equipment (PPE) be worn by carers to minimize the risk of potential transmission of SARS-CoV-2 irrespective of the patient having no symptoms of COVID-19 (Table 3). Recommendations on safety measures to be followed in the home setting is provided in Table 2 (Recommendation 3).

Full table
Safety measures specific to hospital settings
Nebulization in patients with suspected/confirmed COVID-19 must be carried out in a negative pressure room where possible. If such a facility is unavailable, a dedicated area for carrying out nebulization must be set up within the hospital. In the case of patients with no COVID-19 symptoms, they must be admitted to areas where no handling of COVID cases is ongoing to avoid the risk of patients inadvertently contracting COVID-19. Nebulizers are not to be shared to avoid cross-infection and therefore, are for single-patient use only (11,53). Attachment of a HEPA filter to the nebulizer has been reported to substantially decrease the amount of exhaled aerosol particles (58,59). As such, we recommend attaching HEPA filters to the nebulizer prior to delivering medication to the patient as effective risk mitigation for the potential transmission of SARS-CoV-2 from the patient to HCPs or carers, if COVID-19 is suspected or confirmed. Use of face masks with nebulizers should be avoided due to the risk of expulsion of aerosols from the equipment during expiration and breath-holding (46,59,60). However, in very young children needing a face mask, face masks creating a tight seal must be used. A mouthpiece should be preferably used with jet and mesh nebulizers, with filters or one-way valves attached to the large-bore tubing of a jet nebulizer to avert fugitive aerosol emissions (46,59,60). A HEPA filter can be connected to the expiratory limb of a T-piece with the mouthpiece connected to the inspiratory limb of the T-piece. In the case of a mesh nebulizer, a filter should be attached to the other end of the mouthpiece to prevent the release of aerosols to the surroundings (46,59,60).
Cleaning and sterilization or disinfection of nebulizers must be carried out for all types of nebulizers (disposable and reusable) (53). This must be based on manufacturer’s instructions and existing standard hospital protocols for the sterilization or disinfection of semi-critical devices. In hospitals with a central processing facility, in addition to existing disinfection protocols pertaining to the current pandemic, nebulizers can be reprocessed using central facilities such as steam sterilization or autoclave, provided the device can be returned to the patient in a timely manner before their next use (53). At any case, the choice of method must comply with the manufacturer’s permitted method to avoid damage to components (53,54). Recommendations on safety measures to be followed in the hospital setting are provided in Table 2 (Recommendation 4).
Safety measures at school
With schools reopening in various parts of the world during this pandemic, it is important to focus on asthma control and adherence to medication in children with asthma (61). Uncontrolled asthma can lead to academic underperformance and could result in children with asthma missing out on education (51,61). It is important for schools to have a well-managed asthma management plan in place to support their students (51). In response to the current pandemic, CDC guidelines for schools include continued use of nebulizer therapy in those children where no other options exist for various aforementioned reasons (13). Since children with suspected or confirmed COVID-19 should not be attending school, our recommendation for risk mitigation is for children who have no COVID-19 symptoms (61). A summary of recommendations on safety measures to be followed in the school setting is provided in Table 2 (Recommendation 5).
Further studies are required to establish the transmission risk of COVID-19 associated with different modes of nebulization
Data on the spread of COVID-19 through different modes of nebulization are very limited. Only few studies have investigated the risk of aerosol-generating therapy resulting in transmission of any coronavirus (10). The WHO guidelines on infection and prevention control of acute respiratory infectious diseases highlight the significant knowledge gap pertaining to AGPs and the risk of transmission of infectious agents (62). The quality of existing data on transmission risk of SARS-CoV-2 via nebulization is rather poor (10). Although a study from 2004 on PCR air sampling around a patient with SARS undergoing nebulizer treatment found no evidence of virus dispersal from a large-volume nebulizer, the study was limited to one patient (63).
The contradictory statements from GINA and NICE on the transmission risk of SARS-CoV-2 during nebulization can be attributed to an absence of robust data. There is a need for data on the transmission risk posed by different nebulizer devices. Studies in this field are required before clear guidance can be issued.
Summary
The main therapy goal for patients with asthma with or without COVID-19 is minimizing exacerbations and achieving asthma control (38). There are significant risks to a child suffering from a severe asthma attack and these attacks can result in fatal outcomes (34). Therefore, any deviation from planned, individualized asthma therapy, including nebulization, should be avoided wherever possible.
Data on whether nebulized treatment represents an infection transmission risk for SARS-CoV-2 are limited. In light of the above, we advocate that nebulization should be continued during the COVID-19 pandemic but with appropriate safety precautions in the following patient populations: pre-school children, children who are currently receiving nebulization as part of their personal asthma plan, children who cannot operate an MDI plus spacer, in children with impaired asthma control when using the MDI plus spacer and those who present with severe exacerbations or are in respiratory distress (acute asthma/wheeze).
In patients with suspected or confirmed COVID-19, it should be assumed that nebulization can transmit SARS-CoV-2 and therefore any personnel administering nebulization and any bystanders must use full PPE. It is imperative to individualize the risk-benefit assessment of nebulization. The risk posed by nebulization should be assessed by the treating physician who should perform a case-by-case risk assessment on an individual basis. HCPs should also consider individual needs, preferences and distinctive characteristics of local healthcare systems. Our recommendations were developed based on literature review and discussion of the evidence base as of March 2021. New data will continue to emerge during the COVID-19 pandemic. We do not anticipate data that would significantly change our recommendations, but we hope future studies will provide additional clarity on specific populations and situations. HCPs should monitor the literature to maintain awareness of data that may be relevant to their decision-making.
Overall, our recommendation is that nebulization should remain the choice of treatment for children requiring this mode of treatment. We hope the safety measures discussed in this study will help guide treating physicians on best practice in pediatric asthma treatment during the pandemic, so that nebulization will not be withheld from patients in need.
Acknowledgments
Yamini Khirwadkar, PhD, of Darwin Healthcare Communications (Oxford, England) acted as the moderator during consensus meetings. Medical writing assistance was provided by Sreerekha S. Pillai, PhD, of Darwin Healthcare Communications (Oxford, England) and was fully funded by Astra Zeneca.
Funding: This study was funded by Astra Zeneca. The study sponsor has no role in the design of the study, data collection and analysis, and data interpretation, and has not contributed to the manuscript content in any manner.
Footnote
Reporting Checklist: The authors have completed the AGREE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-841
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-841). JH has received honoraria for lectures from AstraZeneca and Chiesi; MDCCS has received honoraria for lectures from AstraZeneca, Boehringer Ingelheim, Moksha 8 and Novartis, for participation in advisory board meetings from AstraZeneca, GlaxoSmithKline and Boehringer Ingelheim, has been the director of the Pediatric Department of Latino-American Thoracic Association-ALAT (2019–2020) and is the head of the Asthma Section of the Mexican Society of Pulmonology (2019–2021); SL has been the President of the Thai Society of Pediatric Respiratory and Critical Care Medicine (2015–2019). All the authors report funding from Astra Zeneca.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2020. Available online: https://ginasthma.org/ (Accessed February 18 2021).
- Licskai C, Yang CL, Ducharme FM, et al. Addressing therapeutic questions to help Canadian physicians optimize asthma management for their patients during the COVID-19 pandemic. Canadian Journal of Respiratory, Critical Care, and Sleep Medicine 2020;4:73-6. [Crossref]
- Australian Commission on Safety and Quality in Health Care. COVID-19 position statement - Nebulisation and COVID-19. 2020. Available online: https://www.safetyandquality.gov.au/publications-and-resources/resource-library/covid-19-position-statement-nebulisation-and-covid-19 (Accessed February 18 2021).
- Taillé C, Chenivesse C, Devouassoux G, et al. Management of asthma during the Coronavirus disease 2019 outbreak. Respir Med Res 2020;78:100762 [Crossref] [PubMed]
- Global Initiative for Asthma. GINA guidance about COVID-19 and asthma. 2021. Available online: https://ginasthma.org/wp-content/uploads/2021/03/21_03_30-GINA-COVID-19-and-asthma.pdf (Accessed April 2021).
- Amirav I, Newhouse MT. Transmission of coronavirus by nebulizer: a serious, underappreciated risk. CMAJ 2020;192:E346 [Crossref] [PubMed]
- Public Health Agency of Canada. Annex F. Prevention and control of influenza during a pandemic for all healthcare settings. 2011. Available online: https://www.phac-aspc.gc.ca/cpip-pclcpi/assets/pdf/ann-f-eng.pdf (Accessed February 18 2021).
- Tang JW, Li Y, Eames I, et al. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect 2006;64:100-14. [Crossref] [PubMed]
- Cinesi Gómez C, Peñuelas Rodríguez Ó, Luján Torné ML, et al. Clinical consensus recommendations regarding non-invasive respiratory support in the adult patient with acute respiratory failure secondary to SARS-CoV-2 infection. Rev Esp Anestesiol Reanim (Engl Ed) 2020;67:261-70. [PubMed]
- Hess MW. Nebulized therapy in the COVID-19 era: the right tool for the right patient Int J Chron Obstruct Pulmon Dis 2020;15:2101-2. [Letter]. [Crossref] [PubMed]
- National Institute for Health and Care Excellence. COVID-19 rapid guideline: severe asthma. 2020. Available online: https://www.nice.org.uk/guidance/ng166/resources/covid19-rapid-guideline-severe-asthma-pdf-66141904108741 (Accessed February 18 2021).
- Public Health England. COVID-19: Guidance for maintaining services within health and care settings: infection prevention and control recommendations. 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/954690/Infection_Prevention_and_Control_Guidance_January_2021.pdf (Accessed February 18 2021).
- CDC. FAQs for administrators, teachers, and parents. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/downloads/FAQ-schools-child-care.pdf (Accessed February 18 2021).
- Welch MJ. Nebulization therapy for asthma: a practical guide for the busy pediatrician. Clin Pediatr (Phila) 2008;47:744-56. [Crossref] [PubMed]
- Welch MJ, Martin ML, Williams PV, et al. Evaluation of inhaler device technique in caregivers of young children with asthma. Pediatric Allergy, Immunology, and Pulmonology 2010;23:113-20. [Crossref]
- Chong-Silva DC, Pastorino AC. Practical guide to aerosol therapy in children and adolescents: Joint document from the Brazilian Association of Allergy and Immunology and the Brazilian Society of Pediatrics. Arquivos de Asmas Alergia e Imunologia 2020;4:11.
- Boe J, Dennis JH, O'Driscoll BR, et al. European Respiratory Society Guidelines on the use of nebulizers. Eur Respir J 2001;18:228-42. [PubMed]
- Wang XF, Hong JG. Management of severe asthma exacerbation in children. World J Pediatr 2011;7:293-301. [Crossref] [PubMed]
- Furman EG, Khuzina EA, Repetskaya MN. Bronchial asthma in children amidst the novel coronavirus infection. DoctorRu 2020;19:42-7. [Crossref]
- Benge CD, Barwise JA. Aerosolization of COVID-19 and contamination risks during respiratory treatments. Fed Pract 2020;37:160-3. [PubMed]
- Moloney E, O'Sullivan S, Hogan T, et al. Airway dehydration: a therapeutic target in asthma? Chest 2002;121:1806-11. [Crossref] [PubMed]
- Nicolini G, Cremonesi G, Melani AS. Inhaled corticosteroid therapy with nebulized beclometasone dipropionate. Pulm Pharmacol Ther 2010;23:145-55. [Crossref] [PubMed]
- Geppe NA, Kolosova NG, Zaytseva OV, et al. Diagnostic and treatment of bronchial asthma in children of preschool age. Place of nebulized inhaled glucocorticosteroids in treatment of bronchial asthma and croup (consensus on the results of the council of experts of the Pediatric Respiratory Society). Russian Bulletin of Perinatology and Pediatrics 2018;63:124-33. [Crossref]
- Usmani OS. Inhaled drug therapy for the management of asthma. Prescriber 2015;26:23-5. [Crossref]
- Shen K, Deng L, Li Y et al. Expert consensus on the application of corticosteroid inhalation therapy in pediatrics (2018 revised edition). Journal of Clinical Pediatrics 2018;36:95-107.
- Murphy KR, Hong JG, Wandalsen G, et al. Nebulized inhaled corticosteroids in asthma treatment in children 5 years or younger: a systematic review and global expert analysis. J Allergy Clin Immunol Pract 2020;8:1815-27. [Crossref] [PubMed]
- McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm 2016;38:655-62. [Crossref] [PubMed]
- Harding H, Broom A, Broom J. Aerosol-generating procedures and infective risk to healthcare workers from SARS-CoV-2: the limits of the evidence. J Hosp Infect 2020;105:717-25. [Crossref] [PubMed]
- Tran K, Cimon K, Severn M, et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012;7:e35797 [Crossref] [PubMed]
- Abrams EM, Sinha I, Fernandes RM, et al. Pediatric asthma and COVID-19: the known, the unknown, and the controversial. Pediatr Pulmonol 2020;55:3573-8. [Crossref] [PubMed]
- Tashkin DP, Barjaktarevic IZ. Nebulized treatments and the possible risk of coronavirus transmission: where is the evidence? Chronic Obstr Pulm Dis 2020;7:136-8. [Crossref] [PubMed]
- NSW Government. Aerosol generating respiratory therapies: nebulisers. 2020. Available online: https://www.health.nsw.gov.au/Infectious/covid-19/communities-of-practice/Pages/guide-respiratory-nebulisers.aspx (Accessed February 18 2021).
- Direkwattanachai C, Aksilp C, Chatchatee P, et al. Practical considerations of nebulized corticosteroid in children with acute asthmatic exacerbation: a consensus. Asian Pac J Allergy Immunol 2019; Epub ahead of print. [Crossref] [PubMed]
- Nagakumar P, Davies B, Gupta A. Acute asthma management considerations in children and adolescents during the COVID-19 pandemic. Arch Dis Child 2021;106:e31 [Crossref] [PubMed]
- Sethi S, Barjaktarevic IZ, Tashkin DP. The use of nebulized pharmacotherapies during the COVID-19 pandemic. Ther Adv Respir Dis 2020;14:1753466620954366 [Crossref] [PubMed]
- Oliver D. David Oliver: could we do better on hospital acquired covid-19 in a future wave? BMJ 2021;372: [PubMed]
- Richterman A, Meyerowitz EA, Cevik M. Hospital-acquired SARS-CoV-2 infection: lessons for public health. JAMA 2020;324:2155-6. [Crossref] [PubMed]
- Hasan SS, Capstick T, Zaidi STR, et al. Use of corticosteroids in asthma and COPD patients with or without COVID-19. Respir Med 2020;170:106045 [Crossref] [PubMed]
- Brough HA, Kalayci O, Sediva A, et al. Managing childhood allergies and immunodeficiencies during respiratory virus epidemics - The 2020 COVID-19 pandemic: a statement from the EAACI-section on pediatrics. Pediatr Allergy Immunol 2020;31:442-8. [Crossref] [PubMed]
- Ferrante G, La Grutta S. Reasons for inadequate asthma control in children: an important contribution from the "French 6 Cities Study". Multidiscip Respir Med 2012;7:23. [Crossref] [PubMed]
- Lenney W, Bush A, Fitzgerald DA, et al. Improving the global diagnosis and management of asthma in children. Thorax 2018;73:662-9. [Crossref]
- Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020;582:557-60. [Crossref] [PubMed]
- HSE (UK). Rapid evidence review: delivered by HSE for the government Chief Scientific Adviser. 2020. Available online: https://www.hse.gov.uk/coronavirus/assets/docs/face-mask-equivalence-aprons-gown-eye-protection.pdf (Accessed March 19 2021).
- World Health Organization. Infection prevention and control during health care when coronavirus disease (COVID-19) is suspected or confirmed. 2020. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC-2020.4 (Accessed March 19 2021).
- CDC. When and how to wash your hands. 2020. Available online: https://www.cdc.gov/handwashing/when-how-handwashing.html (Accessed February 18 2021).
- Cazzola M, Ora J, Bianco A, et al. Guidance on nebulization during the current COVID-19 pandemic. Respir Med 2021;176:106236 [Crossref] [PubMed]
- Fink JB, Ehrmann S, Li J, et al. Reducing aerosol-related risk of transmission in the era of COVID-19: an interim guidance endorsed by the International Society of Aerosols in Medicine. J Aerosol Med Pulm Drug Deliv 2020;33:300-4. [Crossref] [PubMed]
- PHE Transmission Group. Factors contributing to risk of SARS-CoV2 transmission associated with various settings. 2020. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/945978/S0921_Factors_contributing_to_risk_of_SARS_18122020.pdf (Accessed March 17 2021).
- Irish Thoracic Socitey. Guidelines for use of nebuliser systems in the home environment. 2018. Available online: https://irishthoracicsociety.com/wp-content/uploads/2017/05/Nebuliser-Guidelines.pdf (Accessed March 19 2021).
- CDC-HIPAC. Disinfection and sterilization: summary of recommendations. 2019. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/index.html (Accessed March 19 2021).
- National Institute of Health (US). Managing asthma: a guide for schools. 2014. Available online: https://www.nhlbi.nih.gov/files/docs/resources/lung/NACI_ManagingAsthma-508%20FINAL.pdf (Accessed March 19 2021).
- CDC. Guideline for disinfection and sterilization in healthcare facilities, 2008. 2019. Available online: https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf (Accessed April 7 2021).
- O'Malley CA. Device cleaning and infection control in aerosol therapy. Respir Care 2015;60:917-27; discussion 928-30. [Crossref] [PubMed]
- Cystic Fibrosis Foundation. Nebulizer care at home. Available online: https://www.cff.org/Life-With-CF/Treatments-and-Therapies/Medications/Nebulizer-Care-at-Home/ (Accessed March 19 2021).
- CDC. People with moderate to severe asthma. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/asthma.html (Accessed March 19 2021).
- Alexander L, Carson J, McCaughan J, et al. Thinking inside the box: nebulizer care, safe storage, and risk of infection in cystic fibrosis. J Bras Pneumol 2020;46:e20190226 [Crossref] [PubMed]
- SAGE Environment and Modelling Group. Role of aerosol transmission in COVID-19. 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/907587/s0643-nervtag-emg-role-aerosol-transmission-covid-19-sage-48.pdf (Accessed March 12 2021).
- Wittgen BPH, Kunst PWA, Perkins WR, et al. Assessing a system to capture stray aerosol during inhalation of nebulized liposomal cisplatin. J Aerosol Med 2006;19:385-91. [Crossref] [PubMed]
- Swarnakar R, Gupta NM, Halder I, et al. Guidance for nebulization during the COVID-19 pandemic. Lung India 2021;38:S86-91. [Crossref] [PubMed]
- Ari A. Practical strategies for a safe and effective delivery of aerosolized medications to patients with COVID-19. Respir Med 2020;167:105987 [Crossref] [PubMed]
- Abrams EM, McGill G, Bhopal SS, et al. COVID-19, asthma, and return to school. Lancet Respir Med 2020;8:847-9. [Crossref] [PubMed]
- World Health Organization. Infection prevention and control of epidemic-and pandemic prone acute respiratory infections in health care. 2014. Available online: https://www.who.int/publications/i/item/infection-prevention-and-control-of-epidemic-and-pandemic-prone-acute-respiratory-infections-in-health-care (Accessed February 18 2021).
- Wan GH, Tsai YH, Wu YK, et al. A large-volume nebulizer would not be an infectious source for severe acute respiratory syndrome. Infect Control Hosp Epidemiol 2004;25:1113-5. [Crossref] [PubMed]


