|
Original Article
Treatment Failure after Extrapleural Pneumonectomy for Malignant Pleural Mesothelioma
Tristan D Yan, MoMo Tin, Michael Boyer, Jocelyn McLean, Paul G. Bannon, Brian C McCaughan
From the University of Sydney (Drs Y an, McLean, Bannon and McCaughan), Department of Cardiothoracic Surgery, Royal Prince A lfred Hospital; the Baird
Institute for Applied Heart and Lung Surgical (Drs Y an, McLean, Bannon and McCaughan), A ustralia; Department of Medical Oncology (Drs Tin and Boyer),
Royal Prince A lfred Hospital; Sydney Cancer Center (Drs Tin and Boyer), Australia; Department of Radiation Oncology, Royal Prince A lfred Hospital, Sydney, Australia
Corresponding to: Tristan D. Yan, BSc (Med), MBBS, PhD. University of Sydney, Department of Cardiothoracic Surgery, Royal Prince Alfred Hospital, Sydney, Australia. Tel: +61-2-95501933; Fax: +61-2-95506669. E-mail: tristan.yan@unsw.edu.au
|
|
Abstract
Background:Extrapleural pneumonectomy (EPP) has been used as a treatment option for selected patients with malignant pleural mesothelioma (MPM). The primary end-point of this study was disease-free survival (DFS). Prognostic indicators for local and overall DFS were
statistically analyzed.
Methods: Between October 1994 to April 2008, 59 patients who had complete macroscopic cytoreduction after EPP formed the basis of
this report. In recent years, selected patients received adjuvant radiotherapy and pemetrexed combined with cisplatin or carboplatin. The
clinicopathologic data of all patients were prospectively collected in a computerized database. Statistical analysis was performed by using
Kaplan-Meier method and compared using the log-rank test. Cox-regression model was used for multivariate analysis.
Results: The mean age at the time of EPP was 59 (S.D. = 8) years. Nineteen patients (32%) experienced perioperative complications. The
median survival was 21 months (range 2 to 104). The local disease recurrence rate was 51%. The median local DFS was 22 months (0 to
73). The overall disease recurrence rate was 64%. The median overall DFS was 18 months (range 0 to 73). In multivariate analysis, epithelial subtype (p = 0.026) and adjuvant radiotherapy (p = 0.023) were independently associated with an improved local DFS. Adjuvant radiotherapy (p = 0.011) was also independently associated with an improved overall DFS.
Conclusions: This study demonstrated that that local disease failure was still a considerable clinical problem following complete EPP. The
data also showed that patients with epithelial histology and receiving adjuvant radiotherapy were associated with an improved disease control.
Key words
pleural mesothelioma; extrapleural pneumonectomy; radiotherapy
J Thorac Dis 2009;1:23-28. DOI: 10.3978/j.issn.2072-1439.2009.12.01.017
|
|
Introduction
Malignant pleural mesothelioma (MPM) is an aggressive cancer
for which treatment options are limited. The clinical course is
marked by relentless local growth of the tumor, with patients'
deaths most commonly due to cardiac or pulmonary involvement.
In its early stages, MPM remains localized to a single hemithorax
and therapeutic efforts have therefore focused on local treatment
modalities, be it surgical resection, radiotherapy and intrapleural
chemotherapy ( 1- 4). Extrapleural pneumonectomy (EPP) is a radical surgical approach, which can potentially eradicate macroscopic
disease in selected patients ( 5). The perioperative outcome of EPP
has improved significantly in recent years, because of better surgical
techniques and perioperative care ( 6, 7). However, the
long-term survival is still unsatisfactory due to high incidence of
recurrence, especially locoregional treatment failure, at the site
of the surgeons' best effort. Recent evidence suggests that improvements in locoregional disease control have occurred
through the addition of high dose hemithorax radiation and
more potent chemotherapeutic regimens to EPP ( 3, 8- 10). The
multimodality approach to surgically eradicate gross disease followed by additional radiotherapy and/or chemotherapy to control
residual microscopic disease has a strong locoregional treatment
rationale.
In the current literature, the clinical evidence for disease recurrence after EPP is still limited and the predictors of local disease
failure and their impact on subsequent outcome have not been
clearly defined. To address these issues, the outcomes for MPM
patients who underwent EPP were evaluated. The primary
end-point of this study was disease-free survival (DFS), determined
from the time of EPP intervention. Prognostic indicators for local
and overall DFS were statistically analyzed.
|
|
Materials and methods
Between October 1994 to April 2008, 424 patients with a tissue
diagnosis of MPM were treated by a thoracic surgical team, lead by
the same surgeon (B.C.M.). Seventy patients (17%) were selected
for EPP. The criteria for EPP were the extent of disease limited to
the ipsilateral hemithorax with no transdiaphragmatic, transpericardial
or extensive chest wall involvement, a good performance status,
normal renal and liver function tests, adequate cardiac and pulmonary
function assessment. Informed consent was obtained from
all patients prior to surgery. Four patients who died within the
same hospital admission were excluded from this study. Another
seven patients who had positive macroscopic resection margin
were also excluded. The remaining 59 patients who had complete
macroscopic cytoreduction after EPP formed the basis of this report.
The clinicopathologic data of all patients were prospectively
collected in a computerized database.
Preoperative assessment
Preoperative assessment included a review of all prior clinical
information, physical examination, serum chemistry and hematology,
chest X-ray, computed tomography (CT) of the chest and upper
abdomen and pulmonary function testing. Since the year 2000,
positron emission tomography (PET) became available at our institution.
A few patients received preoperative pemetrexed combined
with cisplatin or carboplatin prior to EPP.
Operative techniques
EPP was performed with en bloc resection of the lung, pleurae,
ipsilateral hemi-diaphragm and pericardium. EPP was approached
from an extended posterolateral thoracotomy incision at entire
costal surface of the lung and extending over the apex of the pleura,
mobilizing mediastinal pleura down to the hilum. The main pulmonary vessels were
ligated and divided separately and the bronchus was stapled. Upon control of these
structures it is possible to continue the dissection anteriorly by entering the
pericardium and proceed to the resection of the pericardium and the hemi-diaphragm,
en bloc with the lung and the parietal pleura. Systematic mediastinal lymph node dissection
was routinely performed and the specimens were submitted for histological examination.
The pericardial and diaphragmatic defects were repaired
with 2 mm Gore-Tex dual mesh?.
All tissue specimens were submitted for histopathological examination.
Postoperative management
Adjuvant radiotherapy following EPP was introduced in 2002,
in an attempt to improve locoregional disease control ( 3, 10).
Patients were referred to a radiation oncologist for assessment within
6 weeks of surgery. Selection criteria for radiotherapy include good
performance status, adequate residual cardio-pulmonary function
and satisfactory recovery from surgery. Radiotherapy would commence within 12 weeks of surgery. In most of the patients, a
four-beam mixed photon and electron technique was employed,
delivering a total dose of 45 Gy in 25 daily fractions to the entire
hemithorax, ipsilateral mediastinum bed and ipsilateral chest wall.
Chemotherapy was not routinely used as an adjuvant therapy.
However, in recent years some evidence suggested that pemetrexed
plus cisplatin or carboplatin resulted in superior survival time
( 11- 13). In the present study cohort, a proportion of patients received pemetrexed combined with cisplatin or carboplatin.
Follow-up and statistical analysis
All patients were followed prospectively at three-monthly intervals for the first year and six-monthly thereafter until the last time
of contact or death. The follow-up review included clinical examination and assessment of chest CT scans. If indicated abdominal
CT scans were used to detect any concurrent extrathoracic recurrence. The follow-up status was regularly updated in the database
for each patient by a data manager.
The statistical analyses of 11 potential prognostic factors used
local DFS and overall DFS as the primary end-points, determined
from the time of EPP. Disease recurrence was defined as clinical or
radiologic evidence of tumor with or without symptoms. Local disease recurrence was referred to disease recurrence in the ipsilateral
chest wall, where EPP was performed. Overall disease recurrence
was referred to disease recurrence in lymph nodes, contralateral
chest, abdomen and any other systemic site ( 14). These prognostic
factors included age, gender, self-reported prior occupational asbestos exposure, left side versus right of disease, histopathologic
subtype, presence versus absence of lymph nodes, whether preoperative PET was performed, whether preoperative chemotherapy
was given, presence versus absence of perioperative morbidity and
whether postoperative radiotherapy and/or pemetrexed-based
chemotherapy regimens were given. Univariate analysis was performed by using Kaplan-Meier method and compared using the
log-rank test. For multivariate analysis, a Cox-regression (Cox proportional hazards model)
with forward stepwise selection of covariates and with entering and removing limits of p < 0.10 and p >
0.05 was used. The statistical analyses were performed using SPSS
for Windows (Version 14.5; SPSS GmbH, Munich, Germany). A
significant difference was assumed for p < 0.05.
|
|
Results
Patients characteristics
All 59 patients had complete macroscopic cytoreduction following EPP. The follow-up was complete, with a median follow-up of
14 months (range 2 to 104). The mean age at the time of EPP was
59 (S.D. = 8) years. There were 47 (80% ) male patients. Thirty-nine patients (66%) had prior occupational asbestos exposure.
Twenty-nine patients (49%) had left-side and 30 patients (51%)
had right-side EPP. Forty-nine patients (83%) had epithelial and 10
patients (17%) had biphasic or sarcomatoid tumors. Twenty-two patients (37% ) had lymph node metastasis. Thirty-seven patients
(67%) had preoperative PET. Five patients (9%) had preoperative
pemetrexed chemotherapy. Twenty-four patients (41% ) received
adjuvant ipsilateral radiotherapy and 12 patients (20% ) received
pemetrexed combined with either cisplatin or carboplatin postoperatively. Nineteen patients (32%) experienced perioperative complications. In the descending order of frequency, these adverse events
included atrial fibrillation (n = 5), empyema (n = 4), pleural effusion (n = 3), right-side heart failure (n = 2), hemothorax (n = 1),
bronchopulmonary fistula (n = 1), constrictive pericarditis (n = 1),
hydropneumothorax (n = 1) and small bowel herniation through
chest wall (n = 1).
Overall survival and disease-free survival
The median survival was 21 months (range 2 to 104), with actuarial 1-, 2-, 3- and 5-year survival of 67%, 45%, 32% and 15%, respectively. Twenty-two patients (37%) remained alive at the last
time of follow-up. The local disease recurrence rate was 51% (n =
30), with 1- and 2-year local DFS of 67% and 40%, respectively.
The local disease recurrence rate was 29% of patients who were fit
enough to tolerate adjuvant radiotherapy. Ten patients (17%) recurred within 6 months; 18 patients (31% ) recurred within 12
months and 28 patients (47%) recurred within 24 months. The median local DFS was 22 months (0 to 73). The overall disease recurrence rate was 64% (n = 38), with 1- and 2-year overall DFS of
63% and 30% , respectively. The median overall DFS was 18
months (range 0 to 73). Table 1 demonstrates the patterns of disease recurrence after EPP in these 59 patients. It shows that the
most common site of treatment failure was in the ipsilateral
hemithorax (51%), followed by abdomen (12%) and contralateral
hemithorax (7%).

Factors affecting local disease-free survival
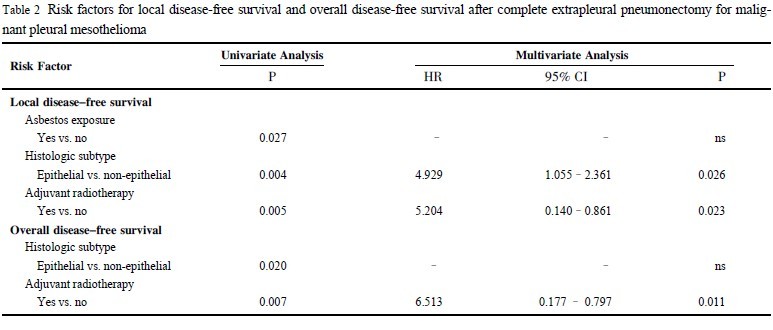
Eleven clinicopathologic factors were analyzed for their prognostic significance in local DFS. Three clinicopathologic factors
were found to be associated with an improved local DFS in univariate analysis: prior occupational asbestos exposure (p = 0.027),
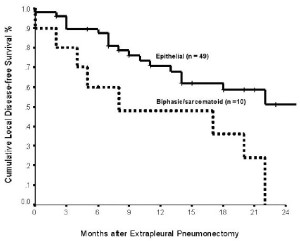
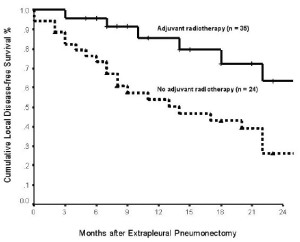
epithelial histologic subtype (p = 0.004) ( Figure 1) and adjuvant
radiotherapy (p = 0.005) ( Figure 2) ( Table 2). Age at the time of
surgery (p = 0.880), gender (p = 0.183), side of disease (p = 0.628),
lymph node involvement (p = 0.288), PET scan (p = 0.081), perioperative morbidity (p = 0.722),
preoperative pemetrexed chemotherapy (p = 0.658) and postoperative pemetrexed combination
chemotherapy (p = 0.117) were not significant prognostic indicators for local DFS in the univariate analysis. In multivariate analysis, epithelial subtype (hazard ratio: 4.929; 95% confidence interval: 1.055 - 2.361; p = 0.026) and adjuvant radiotherapy (hazard
ratio: 5.204; 95% confidence interval: 0.140 - 0.861; p = 0.023)
were independently associated with an improved local DFS.
Factors affecting overall disease-free survival
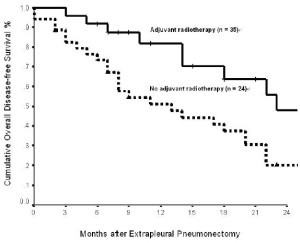
Two clinicopathologic factors were found to be associated with
an improved overall DFS in univariate analysis: epithelial histologic subtype (p = 0.020) and adjuvant radiotherapy
(p = 0.007) ( Figure 3) ( Table 2). Age at the time of surgery (p = 0.918), gender (p= 0.086), asbestos exposure (p = 0.088), side of
disease (p =0.608), lymph node involvement (p = 0.117), PET scan (p =0.102), perioperative morbidity (p = 0.434), preoperative
pemetrexed chemotherapy (p = 0.912) and postoperative pemetrexed
combination chemotherapy (p = 0.201) were not significant prognostic indicators for overall DFS in the present series.
In multivariate analysis, only adjuvant radiotherapy (hazard ratio: 6.513; 95%
confidence interval: 0.177 - 0.797; p = 0.011) was independently
associated with an improved overall DFS.
|
|
Discussion
In the United States, data from Surveillance, Epidemiology and
End Results (SEER) showed a steep rise in the MPM incidence
through the 1990s, with a recent leveling off of the rate of increase,
but no evidence that the peak incidence has been passed in this
country ( 15). Much higher incidence rates are seen in the United
Kingdom and Australia due to the widespread use of asbestos, a
potent inducer of mesothelioma ( 16). The incidence is expected to
continue to increase in areas of the world, where asbestos use has
not been curtailed ( 17). There is substantial public interest in recent
years, because millions of people have been exposed to asbestos in
the environment, especially the workplace. The association with
MPM has created considerable medical-legal implications involving billions of dollars in compensation costs for industry and government.
MPM is a locally aggressive disease. Complete macroscopic cytoreduction can only be achieved with EPP.
It begins with exposure of the parietal pleura, followed by its dissection from the chest
wall, diaphragm and mediastinum. Mediastinal node dissection is
performed, followed by en bloc resection of the diaphragm, pericardium, lung and pleura.
However, despite the best surgical efforts, the cancer recurs and patients eventually die from disease
progression. Many centers have combined EPP with adjuvant radiotherapy and/or systemic chemotherapy ( 3, 8- 10). One of the advantages with EPP is that the lung has been removed. Consequently, radiation toxicity is not as limiting as it is for pleurectomy/decortication, and higher does can be delivered. Patients who
can undergo complete cytoreductive surgery and tolerate adjuvant
chemoradiation have experienced improved median survival as
compared with historical controls. The present study demonstrated locoregional recurrence is the
most common cause of treatment failure after complete EPP. The
local disease recurrence rate was 51% and the median local DFS
was 22 months. This could be related to the presence of microscopic residual disease and/or biological aggressiveness of the tumor.
Baldini and co-workers studied the patterns of failure after EPP
with or without adjuvant chemoradiotherapy and found a recurrence rate of 54% and a median time to first failure of 19 months
( 18). Stewart and colleagues reported a local disease recurrence
rate of 68% and a median time to local disease progression of 21
months after EPP ( 19). However, it has to be acknowledged that a
direct comparison of recurrence rates between series is difficult as
patient characteristics and treatment protocols may differ.
One of the objectives of this study was to identify clinical and
treatment-related data that influence the risk of treatment failure.
The univariate analysis indicated that a short local DFS was associated with absence of prior occupational exposure of asbestos,
non-epithelial histologic subtype and not receiving adjuvant radiotherapy. The association between the absence of prior occupational
exposure of asbestos and reduced local DFS is unclear and this
prognostic factor fell out in the multivariate analysis. A possible
explanation of this correlation is related to both the absence of an
appreciable threshold for asbestos-induced mesothelioma and the
fact that MPM can occur with very low-level exposure ( 20, 21). It
has been hypothesized that some patients may have higher inherent
susceptibility and these susceptible individuals may suffer from
more aggressive disease ( 22). Outside this high susceptibility
group, there may be a dose-response relationship between asbestos
fiber burden and the prognosis ( 22). This paper confirmed that patients with sarcomatoid or biphasic
histologic subtypes fared as a group less well than patients with epithelial histology. Stewart et al. also showed that in patients who
underwent EPP, epithelial histology was associated with delayed
disease progression when compared with biphasic subtype ( 19).
Sugarbaker and colleagues identified that the individuals with epithelial histology who had no positive lymph nodes and complete
EPP achieved a 46% estimated 5-year survival ( 2). A limitation of
EPP surgery from a surgical oncologic standpoint has been that the
margin of resection is usually a single tissue plane, which means
that with an aggressive histology, EPP may be insufficient to result
in a reliable cure.
Adjuvant radiotherapy after EPP was described by the Brigham
group in 1997 ( 18). Thirty-five of 49 patients were given four to
six cycles of postoperative chemotherapy followed by radiation
(median dose 31 Gy). Sixteen irradiated patients developed local
recurrence ( 18). They noted that many patients developed disease
recurrence in the chest and abdomen despite aggressive therapy.The relapse in the abdomen was hypothesized to result from the
continuity of the chest and peritoneal cavity after resection of the
diaphragm. This establishment of the continuity of the chest and
abdominal cavities presumably allows tumor cells disseminate in
the abdomen. In the present study, although the most common site
of relapse after EPP was the ipsilateral thorax, 12% of patients presented with abdominal recurrence. Encouraging results were reported from Memorial Sloan Kettering Cancer Center. From 1995
to 1998, 54 patients underwent EPP followed by adjuvant radiotherapy (median dose 54 Gy) ( 3). The median survival was 18
months. Only 7 patients (13%) developed locoregional treatment
failure and in contrast, 35 patients (65%) had distant recurrence,
with the peritoneum and contralateral pleural being the most common sites ( 3). The present study demonstrated that adjuvant radiotherapy was associated with an improved local disease control
compared with patients who did not receive radiotherapy. However, outside the setting of a randomized controlled trial, subgroup
analysis comparing the patients with or without adjuvant treatments is fraught with potential biases, in that the beneficial treatment effects may be attenuated by confounding factors, such as patient selection and performance status that were not revealed by
this comparative analysis.
In conclusion, this study demonstrated that that local disease
failure was still a considerable clinical problem following complete
EPP, occurring in 51% of all patients and in 29% of patients who
were fit enough to tolerate adjuvant radiotherapy. The data also offered insights into the predictors of treatment failure following
complete EPP and showed that patients with epithelial histology
and receiving adjuvant radiotherapy were associated with an improved disease control.
|
|
Acknowledgments
The authors thank Catherine Kennedy RM RA, for maintaining the thoracic database.
|
|
References
- Brancatisano R, Joseph M, McCaughan B. Pleurectomy for mesothelioma. Med J Aust 1991;154:455-60.
[LinkOut]
- Sugarbaker DJ, Flores RM, Jaklitsch MT, Richards WG, Strauss GM, Corson JM, et al. Resection margins, extrapleural nodal status and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-65.
[LinkOut]
- Rusch VW, Rosenzweig K, Venkatraman E, Leon L, Raben A, Harrison L, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2001;122:788-95.
[LinkOut]
- Rusch V, Saltz L, Venkatraman E, Ginsberg R, McCormack P, Burt M, et al. A phase II trial of pleurectomy/decortication followed by intrapleural and systemic chemotherapy for malignant pleural mesothelioma. J Clin Oncol 1994;12:1156-63.
[LinkOut]
- Grondin S, Sugarbaker D. Pleuropneumonectomy in the treatment of malignant pleural mesothelioma. Chest 1999;116:450-4.
[LinkOut]
- Sugarbaker DJ, Jaklitsch MT, Bueno R, Richards W, Lukanich J, Mentzer SJ, et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg 2005;129:1202-3.
[LinkOut]
- de Perrot M, McRae K, Anraku M, Karkouti K, Waddell TK, Pierre AF, et al. Risk factors for major complications after extrapleural pneumonectomy for malignant pleural mesothelioma. Ann Thorac Surg 2008;85:1206-10.
[LinkOut]
- Sugarbaker DJ, Heher EC, Lee TH, Couper G, Mentzer S, Corson JM, et al. Extrapleural pneumonectomy, chemotherapy, and radiotherapy in the treatment of diffuse malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1991;102:10-5.
[LinkOut]
- Sugarbaker D, Norberto J. Multimodality management of malignant pleural mesothelioma. Chest 1998;113:61-5.
[LinkOut]
- Yajnik S, Rosenzweig KE, Mychalczak B, Krug L, Flores R, Hong L, et al. Hemithoracic radiation after extrapleural pneumonectomy for malignant pleural mesothelioma. Int J Rad Oncol Biol Physic 2003;56:1319-26.
[LinkOut]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44.
[LinkOut]
- Ceresoli GL, Zucali PA, Favaretto AG, Grossi F, Bidoli P, Del Conte G, et al. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol 2006;24:1443-8.
[LinkOut]
- Castagneto B, Botta M, Aitini E, Spigno F, Degiovanni D, Alabiso O, et al. Phase II study of pemetrexed in combination with carboplatin in patients with malignant pleural mesothelioma (MPM). Ann Oncol 2008;19:370-3.
[LinkOut]
- Rusch V, Piantadosi S, Holmes E. The role of extrapleural pneumonectomy in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1991;102:1-9.
[LinkOut]
- Price B, Ware A. Mesothelioma trends in the United States: an update based on Surveillance, Epidemiology, and End Results Program data for 1973 through 2003. Am J Epidemiol 2004;159:107-12.
[LinkOut]
- Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005;353:1591-603.
[LinkOut]
- Kazan-Allen L. Asbestos and mesothelioma: worldwide trends. Lung Cancer 2005;49:3-8.
[LinkOut]
- Baldini EH, Recht A, Strauss GM, DeCamp MM Jr, Swanson SJ, Liptay MJ, et al. Patterns of failure after trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg 1997;63:334-8.
[LinkOut]
- Stewart DJ, Martin-Ucar A, Pilling JE, Edwards JG, O'Byrne KJ, Waller DA. The effect of extent of local resection on patterns of disease progression in malignant pleural mesothelioma. Ann Thorac Surg 2004;78:245-52.
[LinkOut]
- Hansen J, de Klerk NH, Musk AW, Hobbs MS. Environmental exposure to crocidolite and mesothelioma: exposure-response relationships. Am J Respir Crit Care Med 1998;157:69-75.
[LinkOut]
- Hodgson J, Darnton A. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann Occup Hyg 2000;44:565-601.
[LinkOut]
- Christensen BC, Godleski JJ, Roelofs CR, Longacker JL, Bueno R, Sugarbaker DJ, et al. Asbestos burden predicts survival in pleural mesothelioma. Environ Health Perspect 2008;116:723-6.
[LinkOut]
Cite this article as: Yan TD, Tin MM, Boyer M, McLean J, Bannon PG, McCaughan BC. Treatment Failure after Extrapleural Pneumonectomy for Malignant Pleural Mesothelioma. J Thorac Dis 2009;1:23-28. doi: 10.3978/j.issn.2072-1439.2009.12.01.017
|