Pulmonary carcinoid tumorlet without underlying lung disease: analysis of its relationship to fibrosis
Abstract
Pulmonary carcinoid tumorlet is a rare pathology and appears to be always associated with other lesions such as bronchiectasis and fibrosis. While the cause-effect relationship between tumorlet and the accompanying pathological changes in the surrounding mesenchymal tissue remains to be defined, it has been postulated that pulmonary fibrosis may be the primary pathology underlying the development of tumorlet. In this paper, we present a case where a tumor (<0.5 cm) was detected in the right upper lobe of a 71-year old woman. Cells of the tumor displayed markers characterizing for their neuroendocrine origin. No histological evidence for inflammation, interstitial fibrosis and remodeling of vascular structure was observed. However, immunohistochemistry assay demonstrated a strong production of the profibrotic factors VEGF and TGF-β1 by tumor cells. These findings suggest that carcinoid tumorlet can be an isolated lesion and pulmonary fibrosis that “often co-exists” with tumorlet may be secondary to the paracrine effects of fibrotic growth factors produced by tumorlet.
Key words: Lung; tumorlet; pulmonary fibrosis
Introduction
Carcinoid tumorlets are defined as hyperplasia of neuroendocrine cells that are 5 mm or less in size and lack of mitotic activity and necrosis. In the respiratory system, these tumorlets share the histological, ultra-structural, and immunohistochemical features with carcinoids, and the diagnostic discrimination between the two pathologies solely relies on an arbitrarily determined physical dimension (1). Pulmonary carcinoid tumorlet is always associated with underlying lung disease such as bronchiectasis and fibrosis (2), but the relationship between tumorlet and fibrosis remains to be defined. Some researchers believed that hyperplasia of pulmonary neuroendocrine cells is an adaptive response to hypoxia or a secondary process associated with pulmonary fibrosis (3), while other studies suggested that neuroendocrine cells are involved in the pathogenesis of chronic lung diseases (4). In this report, we present a case of pulmonary tumorlet and our data suggests that fibrosis may be a consequence of the enhanced production of the profibrotic growth factors from tumorlets.
Case history
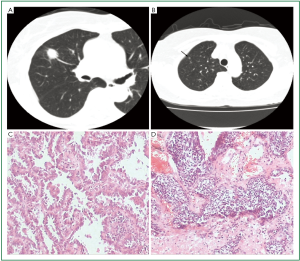
The patient was a 71-year-old woman who was admitted to the Thoracic Surgery Department in July 2009 because a solitary mass was found in her right upper lobe by physical examination. The patient had no symptoms and no smoking history. Computerized tomography demonstrated two nodules in her right upper lobe. The larger nodule located in the anterior segment was 1.8 cm × 1.5 cm, considered lung cancer (Figure 1A), and the smaller nodule located in the apical segment was 0.4 cm × 0.4 cm, considered inflammatory granuloma (Figure 1B). The patient underwent pulmonary lobectomy and mediastinal lymph node dissection.
Materials and methods
The tissue sample for light microscopy was fixed in 10% neutral buffered formalin and processed for routine histologic examination. In addition, sections from the pulmonary tumour were stained with antibodies against Cytokeratin, EMA, TTF-1, CgA, NSE, Syn, CD56, VEGF and TGF-β1 by immunohistochemical analysis using the Super Vision method.
Results
Histologic sections showed two kinds of histological types of tumors in the patient’s lung tissue. The larger nodule was confirmed to be lung invasive adenocarcinoma with predominantly lepidic pattern and 10% papillary pattern (Figure 1C). On the other hand, the smaller nodule was composed of a relatively uniform population of cells with oval or spindle nuclei (Figure 1D). The tumor cells were arranged in nests and cords with finely granular chromatin, inconspicuous nucleoli and moderate amounts of clear cytoplasm. Mitotic activity and necrosis were absent. There was no evidence of airway inflammation, interstitial fibrosis and remodeling of vascular structure in the remaining lung tissue.
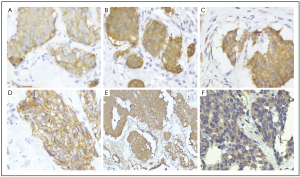
The smaller tumor stained for Cytokeratin, EMA and TTF-1 weakly, but neuroendocrine markers such as CgA, Syn, NSE and CD56 were strongly positive in it (Figure 2A, B, C, D). We diagnosed it as carcinoid tumorlet. Moreover, strongly positive immunostaining for VEGF and TGF-β1 were also found in the tumorlet (Figure 2E, F).
Discussion
Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH), carcinoid tumorlet and typical carcinoid (TC) are a distinct subset of neuroendocrine tumours which share morphologic, ultrastructural, immunohistochemical and molecular characteristics. DIPNECH is defined as a generalized proliferation of scattered single cells, small nodules, or linear proliferations of pulmonary neuroendocrine cells (PNCs) within the bronchial epithelium. The PNCs proliferation may extend beyond the basement membrane into the peribronchial tissue, forming cell nests that are called tumorlets or carcinoids according to their diameter (<0.5 cm or >0.5 cm, respectively) (5). Size is currently the only criteria discriminating tumorlet from typical carcinoid.
Pulmonary carcinoid tumorlets (PCTs) usually constitute incidental findings at autopsy or in pulmonary tissue surgically excised for other reasons (6). PCTs often occur in conditions of chronic lung damage, such as in the context of pulmonary fibrosis, chronic or granulomatous inflammation, bronchiectasis, and giant cell pneumonia. In most instance, neuroendocrine cell hyperplasia and tumorlets are regarded largely as a secondary tissue reaction (7), and in only a few cases suggest that hyperplastic neuroendocrine cells are involved in the pathogenesis of chronic obstructive pulmonary disease and other common airway disorders (8).
In this article, we present a case where both tumorlet and adenocarcinoma were detected in the right upper lobe of a patient without underlying lung disease. Histologically, there was no evidence of airway inflammation, interstitial fibrosis and remodeling of vascular structure in her lung tissue. The case suggests that hypoxia caused by pulmonary fibrosis and airway inflammation may not be the necessary etiological factor for carcinoid tumorlets. There may be other molecular mechanisms involved in the genesis of this tumor.
To study the relationship between cacinoid tumorlet and pulmonary fibrosis, we detected TGF-β1 and VEGF expression in carcinoid tumorlet by immunohistochemistry. TGF-β1, one of the three highly homologous mammalian isoforms (TGF-β1-3), is thought to play a central mediator role in wound healing and fibrosis (9). In addition, a study by HERVE et al. (10) showed that VEGF and its receptors VEGF R1 and VEGF R2 were expressed in tumorlets and in neuroendocrine cell hyperplasia. Their data suggested that VEGF secretion by hyperplastic neuroendocrine cells may contribute to the scarring of lung tissue. Similar to their findings, we also found strong expression of VEGF and TGF-β1 in carcinoid tumorlet of our patient. We presume that pulmonary fibrosis may be a consequence of the enhanced production of the profibrotic growth factors from tumorlets. This presumption could explain why tumorlets were always associated with pulmonary fibrosis in the previous observations.
From a clinical standpoint, PCTs are regarded as benign lesions, although a rare case with metastasis to peribronchial lymph node has been reported (11,12). Without postoperative radiotherapy and chemotherapy, recurrence or metastasis has not occurred in the case of our study.
Acknowledgements
We thank Zhihua Jiang, PhD (University of Florida College of Medicine and the Malcom Randall VAMC), for his valuable suggestions in this article.
Disclosure: The authors declare no conflict of interest.
References
- Aubry MC, Thomas CF Jr, Jett JR, et al. Significance of multiple carcinoid tumors and tumorlets in surgical lung specimens: analysis of 28 patients. Chest 2007;131:1635-43.
- Dewan M, Malatani TS, Osinowo O, et al. Carcinoid tumourlets associated with diffuse bronchiectasis and intralobar sequestration. J R Soc Promot Health 2000;120:192-5.
- Miller RR, Müller NL. Neuroendocrine cell hyperplasia and obliterative bronchiolitis in patients with peripheral carcinoid tumors. Am J Surg Pathol 1995;19:653-8.
- Aguayo SM, Miller YE, Waldron JA Jr, et al. Brief report: idiopathic diffuse hyperplasia of pulmonary neuroendocrine cells and airways disease. N Engl J Med 1992;327:1285-8.
- Vuitch F, Sekido Y, Fong K, et al. Neuroendocrine tumors of the lung. Pathology and molecular biology. Chest Surg Clin N Am 1997;7:21-47.
- Rosai J. An evolutionary view of neuroendocrine cells and their tumors. Int J Surg Pathol 2001;9:87-92.
- Stevens TP, McBride JT, Peake JL, et al. Cell proliferation contributes to PNEC hyperplasia after acute airway injury. Am J Physiol 1997;272:L486-93.
- Davies SJ, Gosney JR, Hansell DM, et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: an under-recognised spectrum of disease. Thorax 2007;62:248-52.
- Lee CG, Kang HR, Homer RJ, et al. Transgenic modeling of transforming growth factor-beta(1): role of apoptosis in fibrosis and alveolar remodeling. Proc Am Thorac Soc 2006;3:418-23.
- Sartelet H, Decaussin M, Devouassoux G, et al. Expression of vascular endothelial growth factor (VEGF) and its receptors (VEGF-R1 [Flt-1] and VEGF-R2 [KDR/Flk-1]) in tumorlets and in neuroendocrine cell hyperplasia of the lung. Hum Pathol 2004;35:1210-7.
- D'Agati VD, Perzin KH. Carcinoid tumorlets of the lung with metastasis to a peribronchial lymph node. Report of a case and review of the literature. Cancer 1985;55:2472-6.
- Dogan R, Kara M, Gundogdu AG, et al. An underrated potential risk of bronchiectasis: lymph node metastasis of a pulmonary tumorlet. Acta Chir Belg 2009;109:109-11.


