
A novel alternative: transapical transcatheter mitral valve-in-valve implantation using J-Valve for failed bioprosthesis
Introduction
In recent years, bioprostheses have been increasingly preferred for valve replacement owing to the favorable clinical results and patient preference (1). In valve replacement surgery, the total number of bioprostheses used surpasses that of mechanical valves used currently (2). However, the Achilles heel of the bioprosthetic valve is the inevitable structural deterioration of valves within 10–20 years (3), which leads to re-intervention. Surgical mitral valve replacement (SMVR) is still the conventional standard therapy for failed mitral bioprostheses. With advancements in surgical techniques and instruments, transcatheter therapy has emerged as a valid alternative to traditional open-heart surgery since the first case of transcatheter aortic valve replacement (TAVR) described by Cribier et al. (4) in 2002. In 2009, Cheung et al. (5) performed the first case of transapical transcatheter mitral valve-in-valve implantation (TM-VIVI) in a human. Here, we describe our experience with transapical TM-VIVI using the J-Valve system for 21 patients with a failed mitral bioprosthesis. Among them, 9 patients with previous aortic-mitral double valve replacement (DVR) were categorized as a special cohort due to the aortic prosthetic valve, which is considered a relative contraindication to TM-VIVI. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-975).
Methods
Patients
From May 2020 to January 2021, 23 consecutive patients were referred to our institution due to mitral bioprosthesis failure and received treatment with transapical TM-VIVI in a hybrid operating room. Nine patients were enrolled while 2 patients were excluded: 1 patient underwent concomitant TAVR and occlusion of mitral perivalvular leakage, and 1 patient underwent concomitant percutaneous coronary intervention. Among the 21 enrolled patients, 8 patients with previous aortic-mitral DVR were categorized as the DVR group while the other 13 patients were categorized as the MVR group. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Guangdong Provincial People’s Hospital (KY-Q-2021-088-01) and informed consent was obtained from all patients.
Preoperative assessment
The preoperative assessment included transthoracic echocardiography (TTE), cardiac computed tomography (CT), and cardiac catheterization. Only 1 patient underwent emergency surgery with incomplete preoperative assessment due to extremely poor general condition, including acute left heart failure, low cardiac output syndrome, and preoperative inserted intra-aortic balloon pump. The most important factor was to determine the true internal diameter of the degenerated bioprosthesis, which depends on the type of valve (stented, stentless, sutureless) and the placement of the leaflets (inside or outside the bioprosthesis). Furthermore, the optimal transcatheter valve size was referred to using the Valve in Valve App (version 2.0, UBQO Limited, London, UK) (6) which contains essential anatomical and fluoroscopic information on available surgical bioprostheses such as the true internal diameter and the suitability and sizes of suggested transcatheter heart valves (THVs). Correct sizing is paramount, as oversizing may affect leaflet mobility resulting in central regurgitation or reduced durability, and undersizing may increase the risk of valve migration or paravalvular regurgitation.
Surgical technique
Transapical TM-VIVI was carried out by an interdisciplinary heart team after patient informed consent was obtained. In all cases, we decided to use the J-Valve, a second-generation self-expandable transcatheter aortic valve implantation device featuring 3 U-shaped graspers that serve as landmarks. The different valve sizes were as follows: 21, 23, 25, 27, and 29 mm.
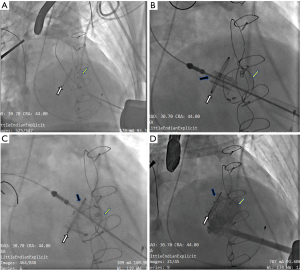
The procedure was performed under general anesthesia and transesophageal echocardiography (TEE) was used for cardiac monitoring. A cardiopulmonary bypass machine was primed and located within the room. A temporary endocardial pacing wire was placed in the right ventricle using cannulation through the right jugular vein. We used fluoroscopy and TEE to determine the location of the left ventricle apex, then the apex was exposed through a left minithoracotomy in the fifth or sixth intercostal space. Through double 3-0 polypropylene purse string sutures reinforced with Teflon pledgets, a guidewire was advanced across the malfunctioning bioprosthesis into a pulmonary vein under fluoroscopy after systemic heparinization. In patients with severe mitral bioprosthesis valve stenosis, balloon valvuloplasty was performed under rapid ventricular pacing (180 times/minute) and ventilation was stopped temporarily. Then, the J-Valve was reversed and crimped onto the delivery device in ice water after determining the optimal size (Figure 1). After replacement of the soft guidewire by an extra-stiff wire guide, the delivery sheath was inserted (Figure 2A). The 3 U-shaped graspers were released in the left ventricle and then advanced toward the sewing ring of the malfunctional bioprosthesis. Subsequently, the reversely crimped J-Valve was positioned inside the malfunctional mitral bioprosthesis (Figure 2B) and was deployed under rapid ventricular pacing (Figure 2C). The sewing ring of the degenerated bioprosthesis was the target for positioning the J-Valve. Post-deployment TEE and angiography were performed to verify no paravalvular or transvalvular leakage and then the delivery sheath was removed (Figure 2D). In 4 patients, TEE revealed mild-moderate paravalvular or transvalvular leakage, so we performed post-deployment dilation to make the new valve totally deploy to fit the malfunctional bioprosthesis leaflets and stent. Protamine was administered, and a left chest tube was inserted with standard closure of the intercostal incision. After the procedure, all patients were treated with warfarin sodium for 3 months, and aspirin thereafter. For patients with contraindications for warfarin sodium, dual antiplatelet therapy was indicated instead.
Endpoint and follow-up
The primary and secondary endpoints were perioperative safety and therapeutic efficacy during the follow-up period, respectively. All patients received TTE assessment before discharge. Clinical evaluation and TTE was performed at 30 days, 6 months, 1 year, and yearly thereafter. The follow-up ended on April 15, 2021.
Statistical analysis
Categorical variables are expressed as frequencies and percentages. Continuous variables are presented as mean ± standard deviation (M ± SD). The Student’s t-test was used to compare continuous variables. Associations between categorical variables were evaluated using the Chi-squared test or Fisher’s exact test as appropriate. A two-sided P value of ≤0.05 was considered statistically significant. Statistical analysis was performed with R (R x64 version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline patient characteristics and hemodynamic data
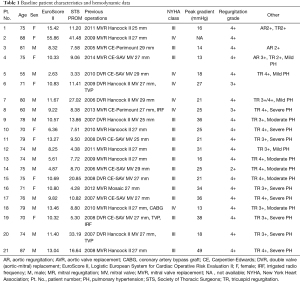
The demographics and hemodynamic data of all patients are listed in Table 1. The mean age was 74.62±7.49 years and males accounted for 57.14% of all patients. All patients were heavily symptomatic with New York Heart Association functional class III or IV. The preoperative risk stratification was assessed according to the Society of Thoracic Surgeons Predicted Risk of Mortality (STS) and European System for Cardiac Operative Risk Evaluation II (EuroScore II), which were 12.03%±10.5% and 12.91%±9.94%, respectively. The previous cardiac operations of all patients included DVR (8 patients) and MVR (13 patients). The failed prosthesis age ranged from 1 to 14 years (11.05±2.36 years), the size ranged from 25 to 31 mm, and the mechanisms of failure were isolated or predominant stenosis in 2 patients, isolated or predominant regurgitation in 17 patients, and mixed in 2 patients. The types of failed bioprostheses were Hancock II, CE-SAV, CE-Perimount, and Mosaic, while the sizes ranged from 25 to 29 mm. Preoperative TTE revealed severe mitral regurgitation in 18 patients and severe mitral stenosis in 3 patients. The preoperative average peak trans-prosthetic gradient was 24.85±9.8 mmHg. Relevant secondary pulmonary hypertension was diagnosed in all patients. Additionally, concomitant moderate to severe regurgitation of the tricuspid valve was detected in 19 patients.
Full table
Procedural data and early outcomes
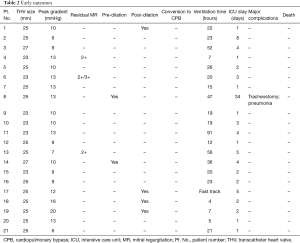
Table 2 summarizes the procedural data and early outcomes of all patients. The procedure was successful in all patients. There was no conversion to median sternotomy and no perioperative death was observed. The mean procedure time averaged 115.80±42.68 minutes. Post-deployment balloon valvuloplasty was required in 4 patients due to trace paravalvular leakage, which may result from the incomplete deployment of transcatheter heart valve.
Full table
The mean time on a ventilator was 25±21.44 hours, and the mean intensive care unit (ICU) length of stay was 4.14±7.08 days. A total of 14 patients were weaned from mechanical ventilation within 24 hours, and 7 patients were transferred to the general ward on the first postoperative day. One patient suffered postoperative pneumonia and tracheostomy, though recovered well and was discharged uneventfully. The main cause may be the preoperative poor pulmonary function with a history of smoking. Only 4 patients needed postoperative blood transfusions. No major complications were observed in the other patients. No perioperative death was observed among all patients. The mean postoperative hospital stay was 9.52±9.76 days. After the operation, all patients were at NYHA class I before discharge. Preoperative and postoperative TTE assessment showed a significant reduction in the peak trans-prosthetic gradient from 24.85±9.8 to 11±3.30 mmHg. Only 3 patients presented with mild mitral regurgitation before discharge.
Follow-up
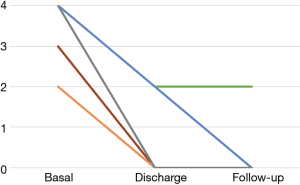
The mean follow-up period was 7.67±2.56 months. Significant symptomatic relief and functional improvement was observed in the majority of patients during follow-up. The hemodynamic performance of the mitral implant was excellent, with an acceptable residual peak gradient of 11±3.30 mmHg. Mild mitral regurgitation was observed only in 1 patient during the follow-up period (Figure 3). All patients were free of symptoms related to cardiac failure at the latest follow-up.
Subgroup analysis
The comparisons of baseline characteristics, procedural data, and early outcomes between the DVR group and MVR group are summarized in Table 3. From the table, it is evident that there is a non-significant difference among all variables between the 2 groups. This indicates that TM-VIVI is also feasible and effective for this special patient group.
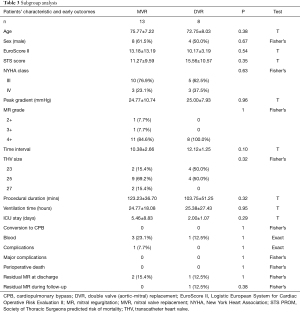
Full table
Discussion
There is an expanding population of high-risk elderly patients who require redo SMVR in parallel with the increasing clinical use of bioprostheses in the treatment of valvular disease. However, conventional SMVR is still associated with a high risk of mortality (4–7%) (7) despite advancements in surgical techniques and instruments. The first reported transapical TM-VIVI by Cheung et al. in 2009 demonstrated its feasibility as an alternative to SMVR. The efficacy and safety of TM-VIVI in the early term and midterm have been reported worldwide (8-10). In previous reports, the most implanted valve was the SAPIEN 3 valve, which is the only THV that is approved for TM-VIVI. In China, however, the SAPIEN 3 valve just received regulatory approval in June 2020 and has not been widely used. Before that, the Venus A-valve, VitaFlow Valve, and J-Valve were the only 3 TAVR systems approved in China.
In this study, we described our experience with transapical TM-VIVI using the J-Valve. The success rate of the procedure was 100% and no death was observed during the study period. Only 1 patient suffered pneumonia and tracheostomy, but recovered well and was discharged uneventfully. All patients returned to normal life with relief of heart failure-related symptoms after the operation. There are several factors that contribute to the excellent clinical outcomes. First, the J-Valve has a shorter stent frame compared to the other valves and it can reduce the risk of left ventricular outflow tract obstruction after implantation. Second, the J-Valve system is a self-expandable TAVR system featuring 3 U-shaped graspers that can capture the degenerated leaflets for anchoring and reduce the risk of displacement after deployment. Unlike prostheses implanted at the position of the aortic valve, the mitral prosthesis can easily be displaced due to the ventricular systolic pressure. Third, the transapical antegrade approach can provide coaxial alignment of the THV into the failed bioprosthesis with the shortest distance and an easier catheter manipulation.
Furthermore, our cases emphasize the possibility of TM-VIVI in patients with previous aortic-mitral DVR. Patients with prior aortic stenosis often have left ventricular hypertrophy and a small left ventricular cavity, which may increase the risk of left ventricular outflow tract obstruction after deployment of the THV. Anchoring of the implanted THV may interfere with the leaflets of the previous aortic prosthesis. In the subgroup analysis, the safety and efficacy of transapical TM-VIVI for patients after aortic-mitral DVR was proven.
As transapical transcatheter mitral valve-in-valve implantation using J-Valve for failed bioprosthesis is still a novel procedure, we acknowledge that there are several limitations, including its small sample size and relatively short-term follow-up period. The long-term outcomes of this procedure require more investigation.
In conclusion, transapical TM-VIVI represents a valid alternative to traditional open-heart mitral valve replacement for patients with a failed prosthesis and also for a special patient group with previous aortic-mitral DVR.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-975
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-975
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-975). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Guangdong Provincial People’s Hospital (KY-Q-2021-088-01) and informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brown JM, O'Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82-90. [Crossref] [PubMed]
- Pibarot P, Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation 2009;119:1034-48. [Crossref] [PubMed]
- Gao G, Wu Y, Grunkemeier GL, et al. Durability of pericardial versus porcine aortic valves. J Am Coll Cardiol 2004;44:384-8. [Crossref] [PubMed]
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. [Crossref] [PubMed]
- Cheung A, Webb JG, Wong DR, et al. Transapical transcatheter mitral valve-in-valve implantation in a human. Ann Thorac Surg 2009;87:e18-e20. [Crossref] [PubMed]
- Bapat V. Valve-in-valve apps: why and how they were developed and how to use them. EuroIntervention 2014;10 Suppl U:U44-51.
- Fatehi Hassanabad A, Turcotte M, Dennehy C, et al. Contemporary Reoperative Mitral Valve Surgery: Technical Considerations and Clinical Outcomes. Innovations (Phila) 2020;15:425-39. [Crossref] [PubMed]
- Yamashita K, Fukushima S, Shimahara Y, et al. Early outcomes of transcatheter mitral valve replacement for degenerated bioprosthesis in Japanese (MITRAL VIV study): a four-case series. Gen Thorac Cardiovasc Surg 2020;68:1-8. [Crossref] [PubMed]
- Whisenant B, Kapadia SR, Eleid MF, et al. One-Year Outcomes of Mitral Valve-in-Valve Using the SAPIEN 3 Transcatheter Heart Valve. JAMA Cardiol 2020;5:1245-52. [Crossref] [PubMed]
- Simonato M, Whisenant B, Ribeiro HB, et al. Transcatheter Mitral Valve Replacement After Surgical Repair or Replacement: Comprehensive Midterm Evaluation of Valve-in-Valve and Valve-in-Ring Implantation From the VIVID Registry. Circulation 2021;143:104-16. [Crossref] [PubMed]



