The efficacy and toxicities of combined lobaplatin with paclitaxel as a first-line chemotherapy for advanced esophageal squamous cell carcinoma
Introduction
Surgery is currently the mainstay of treatment in esophageal cancer. However, the 5-year survival in patients with locally advanced disease managed with surgery alone remains poor, at less than 20-40% (1). Neoadjuvant chemotherapy or chemoradiotherapy (CRT) is recommended as a standard treatment in local advanced esophageal cancer (2,3). To date, no global standard protocol for neoadjuvant or concurrent chemotherapy in esophageal cancer has been established.
Of the commonly used regimens, a combination of 5-fluorouracil with cisplatin or paclitaxel with cisplatin is widely used (4). However, the administration of cisplatin is frequently accompanied by several severe toxicities, including gastrointestinal, nephro- and neurotoxicity. New protocols that can achieve similar outcomes yet minimize the toxicities are required urgently.
Lobaplatin, (D-19466; 1,2-diamminomethyl-cyclobutane-platinum(II)-lactate), characterized by interesting antitumor activity, apparently no nephro- or neurotoxicity and no cross-resistance to cisplatin and carboplatin, demonstrated strong activity against esophageal cancer cell lines in preclinical studies (5). Thus, the efficacy and safety of the combined lobaplatin with paclitaxel (LP) regimen in esophageal squamous cell carcinoma had been reported rarely (6,7), and there was no similar research of the LP when used as first line treatment had been reported.
To assess the efficacy and toxicities of LP for first line treatment of esophageal squamous cell carcinoma we collected and reviewed retrospectively the clinical data of 45 patients.
Methods
Pretreatment workup and eligibility criteria
This retrospective analysis was approved by Fujian Medical University Union Hospital Institutional Review Board. All patients wrote informed consent prior to treatment and all information had been anonymized and de-identified prior to its analysis.
The pretreatment workup included taking patients’ medical history, assessment of swallowing function, physical examination, standard laboratory tests, esophagogastroduodenoscopy, barium esophagography, cervical and abdominal ultrasound, chest computed tomography (CT), bone scan and magnetic resonance imaging. Bronchoscopy was performed if considered necessary. The cTNM stage was determined according to the 7th AJCC TNM staging system (8) by CT scan findings (9).
The eligibility criteria for this retrospective study were as follows: (I) histologically-proven squamous cell carcinoma of the esophagus, age from 18 to 75 years, Eastern Cooperative Oncology Group (ECOG) performance status scoring ≤2; (II) adequate organ function (bone marrow, hepatic, and renal) for chemotherapy (10); (III) local or system advanced tumor and initially chemotherapy with LP at least two cycles; and (IV) patients had an integrity assessment by barium esophagography and CT scan after every two cycles of chemotherapy.
The exclusion criteria included the following: (I) history of other tumors; (II) previous treatment for esophagobronchial or esophagomediastinal fistulas; and (III) other concomitant medical conditions requiring treatment.
Treatment
All patients received at least two cycles of chemotherapy followed with radical local treatment (radiotherapy or surgery) or continuously palliative chemotherapy, which was decided based on the clinical stage, the response and toxicities of chemotherapy and the patients’ wish for treatment. Two to four cycles of adjuvant chemotherapy were conducted within 4 weeks after radical local treatment if conditions permitted.
The dose levels and schedule date of paclitaxel (Jiangsu Yew Pharmaceutical Co. Ltd, Wuxi, China) and lobaplatin (Hainan Changan International Pharmaceutical Co. Ltd, Haikou, China) were 135 mg/m2 dL and 30 mg/m2 dL, respectively, with 3 weeks repeated (11,12). The time interval and dose intensity of chemotherapy were adjusted based on the toxicities of the previous chemotherapy course and the acute toxicities of radiotherapy. The doses were reduced by 25% in the subsequent course if ≥ grade III hematotoxicity, hepatotoxicity, nephrotoxicity, or esophagitis was observed. The treatment was suspended if the recovery from toxicity were delayed >3 weeks.
Patients who received radiotherapy underwent 3-dimensional conformal or intensity modulated radiation therapy. The radiotherapy would normally be initiated 3 weeks after completion of the first two cycles of chemotherapy. The dose and target of radiotherapy were defined as followings (13): the gross tumor volume (GTV) contains the primary tumor and metastasis lymph nodes. The clinical target volume (CTV) was defined as the primary tumor with a margin of 3.0 cm in the cranial-caudal directions for the primary tumor (and a minimum of 1.0 cm for the lymph nodes), a 0.5 cm margin (modified for anatomic boundaries) circumferentially, and the elective lymph nodal regions. The dose to the CTV and GTV was 4,500-5,040 cGy and 5,040-6,600 cGy (180 cGy/fraction), respectively. The dose to CTV and GTV was 4,000 cGy in patients intending to esophagectomy.
The dose limitations of the organ at risk were as followings (14-16): The maximum spinal cord dose was of 4,500 cGy. The lung mean dose was of <1,800 cGy, V20 (volume receiving >2,000 cGy) and V5 (volume receiving >500 cGy) was of <30% (27% in CRT patients) and <70%, respectively. The V45 of the heart was of <60%. For the liver, the V30 was of <60% and the mean dose was of < 3,000 cGy. If any one of organs at risk dose limitations could not be satisfied, the radiotherapy was defined as palliative treatment and was applied to the gross tumor only.
Patients who proceeded to operation underwent thoraco-laparoscopic esophagectomy and extensive two- to three-field lymphadenectomy (17) at 3-4 weeks after completion of the first two cycles of chemotherapy.
To achieve chemotherapy, the prophylactic anti-emetic with a 5-hydroxytryptamine (5-HT3) antagonist was administrated conventional during chemotherapy and the recombinant human granulocyte colony-stimulating factor was commonly administered when patients experienced grade I or more neutropenia. To complete radiotherapy, a low dose of dexamethasone was applied routinely when patients encountered ≥ grade I radiation esophagitis.
Criteria for toxicity, treatment response and dysphagia
The chemotherapy toxicities were graded using the National Cancer Institute common toxicity criteria (NCI CTC v 3.0) (18). The acute RT toxicities were assessed with the RTOG (19).
With the exception of instances where the dysphagia worsened during chemotherapy, the response to chemotherapy was evaluated every two cycles by CT scanning and barium esophagography 3-4 weeks after the completion of chemotherapy and reassessed after at least 4 weeks.
The clinical criteria for response were defined by RECIST1.1 (20) as follows, based on the findings of CT scanning and barium esophagography: a clinical complete response (CR) was defined as no evidence of disease on imaging, negative by endoscopic biopsy or cytology, and free of dysphagia; a partial response (PR) was defined as a 30% or greater decrease in tumor max dimension; and stable disease (SD) was defined as a less than 30% decrease or increase ≤20%, and no evidence of metastatic disease. Progression of disease (PD) was defined as an at least a 20% increase or development of distant metastatic disease. The objective tumor response included the CR and PR.
Dysphagia was evaluated as follows: a score of 0 corresponded to tolerance of normal diet, 1 to some solid food, 2 to semi-solid food only, 3 to liquids only, and 4 to complete inability to swallow (21).
Follow up and statistical analysis
Patients were evaluated every 3 months for the first 2 years after treatment, and future follow-up evaluations are scheduled for every 6 months for the next 3 years, and then annually. Surveillance included interim history and physical examination, assessment of swallowing function, performance status and weight measurement, and laboratory testing. Esophagogastroduodenoscopy and CT of the chest and abdomen were performed every 6 months for 2 years and will be performed annually thereafter.
All patients’ outcomes were evaluated in December 2014.The primary endpoints were overall response and toxicities of chemotherapy. The secondary endpoint was dysphagia relief and toxicities of CRT. The data were analyzed with SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA). The difference of the dysphagia relief was tested by Wilcoxon’s signed rank test. A two-tailed P value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
From January 1, 2013 to July 31, 2014, a total of 45 patients fulfilled the eligibility criteria. The clinical characteristics of these patients are summarized in Table 1.
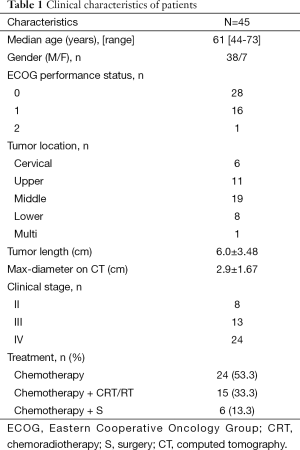
Full table
A total of 117 cycles of LP were delivered to all patients with a median 2.6 courses (range, 2-7 courses). The median follow-up time was 8 months (range, 1-22 months).
Response and toxicities
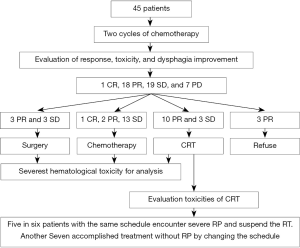
After the first two cycles of chemotherapy, one patient achieved CR, 18 patients reached PR, 19 patients got SD and 7 patients encountered PD. Of patients with CR or PR or SD, 6 patients proceeded to surgery, 16 patients were administrated with palliative chemotherapy, 13 patients scheduled to CRT, 3 patients refused chemotherapy (two patients received RT alone and one patient gave up any more treatment) (Table 2, Figure 1).

Full table
The most common toxicity of chemotherapy was hematological. Gastrointestinal toxicities and hepatotoxicity were very minimal, no nephrotoxicity was observed, and no treatment-related deaths occurred during the study (Table 3).

Full table
A total of 13 patients received CRT were identified for toxicity assessment of radiation. All 13 patients experienced grade I-II radiation esophagitis and no grade III-IV occurred. A grade III/IV radiation pneumonitis were observed in five of the preliminary six patients all treated with the same chemotherapy dose and time interval of concurrent chemotherapy as NAC, and therefore the RT was suspended for these patients. No untolerated lung toxicity recurred in the subsequent CRT patients by extending the time interval of concurrent chemotherapy from 3 to 4 weeks.
Dysphagia relief
All patients had different degree of dysphagia prior to treatment. This resolved or improved in 32 patients (71%), was unchanged in 13 patients (29%) after NAC, and did not worsen in any patient. The improvement of dysphagia after NAC was statistically significant compared to the severity before treatment (Table 4).

Full table
Discussion
Despite the fact that there were no available data from phase III clinical trials, the combined regimen of LP was recommended as one of several second line chemotherapy regimens for advanced esophageal cancer in China (11), and was considered to be a safe and effective protocol for the first line treatment with an overall response (PR and CR) of about 80% (6,7). However, in the present study, the overall response of the LP regimen reached only 42.2% (19/45), similar to the regimen of paclitaxel-cisplatin at 40-45% (22,23) and superior to that of the traditional regimen of 5-fluorouracil with cisplatin at 30-35% (24,25).
Several phase I and II clinical trials indicated that thrombocytopenia is the most common dose-limiting toxicity of lobaplatin (10,26,27). However, in the current study, the most common hematological toxicity of LP was leukopenia in addition to thrombocytopenia. A number of reasonable explanations were that: (I) all patients had initial chemotherapy with LP, the bone marrow and renal function which appeared to correlate with thrombocytopenia was able to recover quickly (10); (II) use of lobaplatin with minimum dose of 30 mg/m2 with only 2.6 courses (range, 2-7 courses) median chemotherapy cycles (28); and (III) the combination of paclitaxel worsened the leukopenia concealing the thrombocytopenia toxicity fact of lobaplatin.
With administration of a 5-HT3 antagonist conventionally during chemotherapy, most of (82.2%) patients experienced mild nausea/vomiting (grade I/II) without grade III-IV instances occurring in the current study, similar to Li’s report of 80.6% (28). In addition, whether treated as first or second line chemotherapy regimen, nothing much of hepatotoxicity and nephrotoxicity were reported previously (6,7,28), as demonstrated in this study, only four patients (8.9%) encountered grade I hepatotoxicity and no nephrotoxicity was observed. All these mild non-hematological toxicities were not required to reduce the dose of treatment and were able to continue on therapy.
Dysphagia is the most common and serious symptom of esophageal cancer. Many prior studies of chemotherapy in esophageal cancer had indicated that chemotherapy by itself might lead to significant palliation of dysphagia (22,23,29). In the current study, regardless of the objective response to neoadjuvant chemotherapy, initial therapy with LP was very effective in dysphagia relief and with no patient worsened. The improvement in dysphagia should be noted and also highlights the importance as a possible palliative therapy. Furthermore, the discrepancy between the dysphagia relief and the objective response suggested that using the improvement of swallowing function as a response evaluation standard for chemotherapy may be not appropriate.
Radiation esophagitis was a common toxicity in esophageal cancer treated with radiotherapy. Lin and his colleague (7) reported firstly on the radiation esophagitis in patients treated with concurrent CRT of LP. In Lin’s study, the grade I/II radiation esophagitis reached up to 88.5% when patients received more than 4 weeks of radiotherapy. The symptomatic pain of the acute radiation esophagitis could relieve by treatment of a low dose of dexamethasone and was considered uncorrelated to the chemotherapy. In our study, though all the 13 patients receiving CRT experienced grade I-II acute radiation esophagitis, there was no severe radiation esophagitis (grad III or IV) had occurred. The above results indicated that the LP had lower mucosa toxicity and would not exacerbate the radiation-induced esophagitis when combined with current radiation therapy.
Radiation pneumonitis is a severe complication in chest radiation therapy. Several factors contribute to this toxicity, including patient’s age, radiation dose-volume of lung, and current chemotherapy regimen and schedule (30). However, there are no comparable reports on radiation pneumonitis following current chemotherapy with LP. Our study is the first to discuss the topic. Of the six patients who received current chemotherapy with the same time interval and dose intensity as the first two cycles chemotherapy in the preliminary treatment, five patients suffered grade III-IV radiation pneumonitis and had to discontinue CRT. To overcome the untolerated radiation pneumonitis, extending the concurrent chemotherapy time interval from 3 weeks to 4 weeks was adopted in the subsequent seven patients and no untolerated lung toxicity recurred. Due to the limited number of cases, the efficacy of such adjustment to prevent radiation pneumonitis requires more researches.
Conclusions
In conclusion, the LP showed a significant antitumor effect to squamous esophageal cancer with main toxicity of leukopenia in addition to thrombocytopenia and mild gastrointestinal, hepatic- or nephro-toxicity toxicities. When combined with radiotherapy, LP would not exacerbate the radiation-induced esophagitis; however, serious lung toxicity should be paid special attention. Limitation of the surveillance time and the retrospective nature, the effect that based on these data formal prospective trials appear warranted and are needed prior to routine first line use of this regimen.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ku GY, Ilson DH. Role of neoadjuvant therapy for esophageal adenocarcinoma. Surg Oncol Clin N Am 2009;18:533-46. [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Kuwano H, Nishimura Y, Oyama T, et al. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus 2015;12:1-30.
- Harstrick A, Vanhoefer U, Heidemann A, et al. Drug interactions of 5-fluorouracil with either cisplatin or lobaplatin--a new, clinically active platinum analog in established human cancer cell lines. Anticancer Drugs 1997;8:391-5. [PubMed]
- Chen JQ, Li JC, Zhu KS, et al. The adverse effects of chemotherapy with lobaplatin and paclitaxel in middle and advanced stage esophageal carcinoma. China Oncology 2012;22:287-290.
- Lin Y, Chen JQ, Li JC, et al. Recent results of concurrent chemoradiotherapy with lobaplatin and paclitaxel in advanced esophageal carcinoma. Cancer research and Clinic 2012;24:105-7.
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Schröder W, Baldus SE, Mönig SP, et al. Lymph node staging of esophageal squamous cell carcinoma in patients with and without neoadjuvant radiochemotherapy: histomorphologic analysis. World J Surg 2002;26:584-7. [PubMed]
- Gietema JA, de Vries EG, Sleijfer DT, et al. A phase I study of 1,2-diamminomethyl-cyclobutane-platinum (II)-lactate (D-19466; lobaplatin) administered daily for 5 days. Br J Cancer 1993;67:396-401. [PubMed]
- He J. The Society of Esophageal Cancer, Chinese Anti-Cancer Association. Clinial Practice Guidelines for the Diagnosis and Treatment of Esophageal Cancer. Beijing, China: Peking Union Medical College Press, 2013:123-40.
- Peng Y, Liu YE, Ren XC, et al. A phase I clinical trial of dose escalation of lobaplatin in combination with fixed-dose docetaxel for the treatment of human solid tumours that had progressed following chemotherapy. Oncol Lett 2015;9:67-74. [PubMed]
- Li QQ, Liu MZ, Hu YH, et al. Definitive concomitant chemoradiotherapy with docetaxel and cisplatin in squamous esophageal carcinoma. Dis Esophagus 2010;23:253-9. [PubMed]
- Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010;76:S77-85. [PubMed]
- Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:S70-6. [PubMed]
- Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys 2010;76:S42-9. [PubMed]
- Lin J, Kang M, Chen C, et al. Thoracolaparoscopy oesophagectomy and extensive two-field lymphadenectomy for oesophageal cancer: introduction and teaching of a new technique in a high-volume centre. Eur J Cardiothorac Surg 2013;43:115-21. [PubMed]
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176-81. [PubMed]
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341-6. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
- Bains MS, Stojadinovic A, Minsky B, et al. A phase II trial of preoperative combined-modality therapy for localized esophageal carcinoma: initial results. J Thorac Cardiovasc Surg 2002;124:270-7. [PubMed]
- Zhang X, Shen L, Li J, et al. A phase II trial of paclitaxel and cisplatin in patients with advanced squamous-cell carcinoma of the esophagus. Am J Clin Oncol 2008;31:29-33. [PubMed]
- Ilson DH, Forastiere A, Arquette M, et al. A phase II trial of paclitaxel and cisplatin in patients with advanced carcinoma of the esophagus. Cancer J 2000;6:316-23. [PubMed]
- Iizuka T, Kakegawa T, Ide H, et al. Phase II evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japanese Esophageal Oncology Group Trial. Jpn J Clin Oncol 1992;22:172-6. [PubMed]
- Bleiberg H, Conroy T, Paillot B, et al. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer 1997;33:1216-20. [PubMed]
- Degardin M, Armand JP, Chevallier B, et al. A clinical screening cooperative group phase II evaluation of lobaplatin (ASTA D-19466) in advanced head and neck cancer. Invest New Drugs 1995;13:253-5. [PubMed]
- Kavanagh JJ, Edwards CL, Freedman RS, et al. A trial of lobaplatin (D-19466) in platinum-resistant ovarian cancer. Gynecol Oncol 1995;58:106-9. [PubMed]
- Li X, Li Y. Clinical study on chemotherapy of lobaplatin combined with docetaxel in patients with relapsed ovarian cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014;39:1131-6. [PubMed]
- Spiridonidis CH, Laufman LR, Jones JJ, et al. A phase II evaluation of high dose cisplatin and etoposide in patients with advanced esophageal adenocarcinoma. Cancer 1996;78:2070-7. [PubMed]
- Dang J, Li G, Zang S, et al. Risk and predictors for early radiation pneumonitis in patients with stage III non-small cell lung cancer treated with concurrent or sequential chemoradiotherapy. Radiat Oncol 2014;9:172. [PubMed]


