Comparison of clinicopathologic features and survival between eastern and western population with esophageal squamous cell carcinoma
Introduction
Approximately 460,000 people are diagnosed with esophageal cancer worldwide annually, with ~380,000 people die of the disease. This makes it the eighth most common cancer and the sixth most common cause of cancer mortality (1). Esophageal squamous cell carcinoma (ESCC) is the major histologic subtype and is characterized by a high mortality rate and geographic differences in incidence (1-3). To date, there has been no comparative study for ESCC between eastern and western countries.
ESCC occurs more commonly in persons of lower socioeconomic status so that the availability of medical treatment is problematic (4). If there are differences in incidence and outcome, it could be as a result of differences in ethnic groups, living conditions, socioeconomic strata, treatment options and choices, and differences in the health care systems (5-9). A recent large genome-wide association study (GWAS) of ESCC has identified two susceptibility genes, PLCE1 and C20orf54, which were highly associated with ESCC in the Chinese population. PLCE1 regulates cell growth, differentiation, apoptosis and angiogenesis. C20orf54 is responsible for transporting riboflavin, and deficiency of riboflavin has been documented as a risk factor for ESCC. PLCE1 and C20orf54 have important biological implications for ESCC and might account for the pathogenesis of ESCC (10). However, to date, no genetic susceptibility to ESCC in Caucasians has been identified.
The goals of our study were as follows: First, to use registry data to provide a detailed picture of ESCC cases, describing gender, age, primary site, stage, grade, and prognosis. Second is to compare ESCC survival for Chinese resident patients and Caucasians living in the US.
Materials and methods
Data source
Data for this analysis came from two sources. Data for Caucasians were from the Surveillance, Epidemiology and End Results (SEER) limited-use database. SEER collects cancer incidence and survival data from population-based cancer registries covering approximately 26% of the U.S. population. The routine data collection includes detailed information on demographics, diagnosis, tumor characteristics and treatment information (11). Data for Chinese were from Shanghai Cancer Registry (SCR) compiled by Shanghai Municipal Center for Disease Control (SMCDC) (12). A total of 175 hospitals, qualified for cancer treatment, in the greater Shanghai area are responsible for reporting all newly diagnosed cancer cases, using a standardized report card. A patient consent form was obtained, following an approved, institutional-reviewed protocol (13). Death certificates for all cancer patients are obtained monthly from the Vital Statistics Section of the SMCDC.
Subjects
From the SEER database, we identified 1,624 Caucasians (tumor site codes 15.0 to 15.5, 15.8, 15.9 and ICD-O-3 morphology code 8070/3), who were diagnosed between January 1st, 2002 and December 31st, 2006, with individual American Joint Commission on Cancer (AJCC) staging information. Similarly, we identified 1,718 Chinese ESCC patients with staging information according to the same criteria. Thus, two groups of patients constitute the subjects of this analysis: Chinese group (1,718 patients) and Caucasian group (1,624 patients). The end follow-up data was December 31, 2009. For the Chinese group, the mean and median follow-up times were 755 and 435 days, respectively (range, 21-1,410 days). For the Caucasian group, the mean and median follow-up times were 725 and 456 days, respectively (range, 29-1,401 days). Like most of population based study, disease-free survival is not available in this study.
Definition of variables
The variables in this analysis included race/ethnicity, gender, age group, tumor stage and primary site. Tumor stage at diagnosis was defined according to the AJCC staging classification, 6th edition. Primary site of the tumor was defined by ICD-O-3 codes and was classified as cervical esophagus (C15.0), upper third of esophagus (C15.3), middle third of esophagus (C15.1, C15.4), lower third of esophagus (C15.2, C15.5), overlapping lesions (C15.8) of esophagus, NOS (C15.9) (2).
Statistical analysis
Differences in the distribution of gender, age group, tumor stage and primary site between two groups were evaluated using the Chi-square test (14).
One-year, 3-year and 5-year overall survival rate and median survival time were calculated using the life-table method. ESCC-specific survival curves were calculated according to the Kaplan-Meier method and compared by the log-rank test (12).
To explain possible survival differences and identify factors affecting survival, the Cox proportional hazards model was used. For each population group, multivariate associations of gender, age group, tumor stage and primary site with survival were determined. The assumption of proportional hazards for Cox models was checked by plotting the log of the negative log of the survival density functions vs. the log of survival time. The plotted lines were roughly parallel over time and no violations of the proportional hazards assumption were found (8).
All analyses were performed with the SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). All P values were two-tailed and P<0.05 was considered statistically significant.
Results
Tumor characteristics of ESCC patients from the two populations
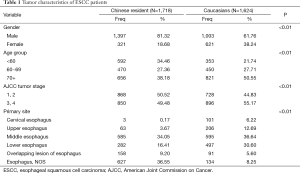
The distribution of cases by gender, age group, primary site and stage, is shown for each population in Table 1. The Caucasian group had a significantly higher proportion of female patients than Chinese (38.24% vs. 18.68%, P<0.01). Chinese patients were younger, with only 31.18% of patient above 70 years old while Caucasians with 50.55% above 70 years (P<0.01). A total of 49.48% patients were diagnosed in the advanced stages (stages III and IV) in the Chinese resident population, while the majority of Caucasians (55.17%) presented with advanced stage disease (P<0.01).
Full table
Survival analysis by age, gender and tumor stage
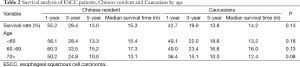
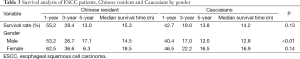
One-year, 3-year and 5-year overall survival rate and median survival time are shown in Table 2. Median survival time was within a narrow range (14.2-15.3 months) in the two populations. Generally, Chinese patients had similar overall survival rate with Caucasians, (median survival time, 15.3 vs. 14.2 months, P=0.40). Overall survival was significantly lower in male (but not female) patients from Caucasians compared to Chinese patients (median survival time, 12.8 vs. 14.5 months, P<0.01, respectively, Table 3). No statistically significant difference in survival was seen among patients of each age group or tumor stage from the two populations (Tables 2-4).
Full table
Full table

Full table
Multivariate analysis in ESCC patients
Among age, sex, stage and, tumor site, age and stage were found to be independent prognostic factors in each population after adjustment using the Cox proportional hazards model (P<0.01).
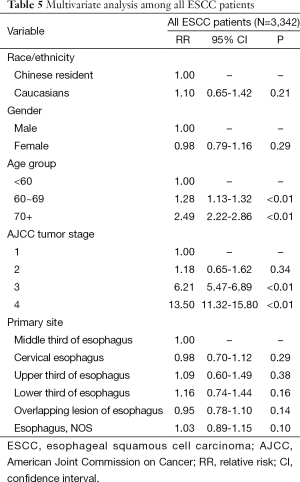
A Cox proportional hazards model built jointly for all ESCC patients suggested no significant advantage in survival of Chinese compared to Caucasian patients [relative risk (RR) =1.10, P=0.21]. Multivariate analysis disclosed age and stage were independent prognostic factors in all ESCC patients (P<0.01, respectively, Table 5).
Full table
Multivariate analysis in all male ESCC patients
Since univariate analysis showed overall survival was significantly lower in male patients from Caucasians compared to Chinese patients, multivariate analysis was further performed to confirm this conclusion. Multivariate analysis among all male ESCC patients suggested age and stage were independent prognostic factors (P<0.01). Compared to male Chinese, Caucasians had a worse prognosis (RR =1.54, P<0.01, Table 6).
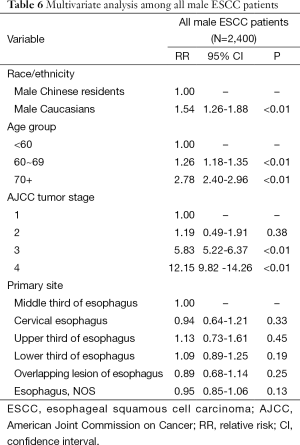
Full table
Discussion
ESCC remains the predominant histological subtype of esophageal cancer world-widely (2,3). However, previous studies had provided scant and discordant data about the differences in tumor characteristics and prognoses between different ethnical groups (15). Several interesting results are disclosed in the current study: Chinese patients were diagnosed with ESCC at an earlier age and stage than Caucasians (Tables 2,6). Another interesting finding was that Chinese patients had similar overall survival rate with Caucasian by both univariate and multivariate analysis. The median survival time of male Chinese patients was significantly longer than that of Caucasians (Tables 3,6).
Compared to Caucasians, Chinese patients develop the disease at an earlier age and earlier stage for the entire population (Table 2) and for males-only (Table 6). This may be attributed to different causes of the disease between the populations. A familial aggregation tendency in high-risk areas suggests that genes may play a role in ESCC pathogenesis (16,17). As mentioned in Introduction, several genes have been considered to contribute to ESCC in Chinese, such as PLCE1 and C20orf54 (10), and ADH1B and ALDH2 in Japanese (18). This genetic susceptibility among Asian populations may partly explain the early onset in Chinese patients. There has been research concerning environmental factors as well. Some studies have demonstrated that smoking, alcohol consumption, and high body mass index (BMI) are more important risk factors of ESCC in western countries than in China (3,19,20). Thus, for the American patients, it would take longer to develop ESCC, reflecting the cumulative effects of these factors. On the other hand, for Chinese, nutritional imbalance, intake of pickled vegetables, nitrosamine-rich and mycotoxin-contaminated foods seem more important (21,22). Such factors may take less time to develop neoplasia.
We also noted that Chinese male patients lived longer than Caucasians (Tables 3,6). Younger patients usually have fewer comorbid diseases, less complications from treatment, may have more of an economic ability to afford treatment, and more willingness to invest in health. Thus, younger patients could have better outcomes if their diseases are not too aggressive (23,24). Besides, a considerable percentage of the western patients get ESCC after radiotherapy on mediastinum for their lymphoma. These tumors resulting from radiation grow with more invasive and malignant biological characteristics. This may be another explanation of the relatively poorer prognoses of Caucasians.
We also noticed the difference in distribution between males and females. In comparison with Chinese, Caucasians had a significantly higher proportion of females (Table 1). This difference may partly be attributed to higher smoking and drinking prevalence among Caucasian women.
The strength of this study is that the analysis of these characteristics and comparing Chinese resident patients and Caucasians living in the US, has not previously been done. However, our study was limited to available variables and comparable databases, so that not all possible factors affecting survival were considered. In addition, socio-economic status, family history of ESCC, and lifestyle factors, such as alcohol consumption and smoking, were also not studied. Further prospective studies are also needed to define the genetic susceptibility for ESCC, especially in western countries.
Acknowledgements
Funding: We acknowledge supported by key technology support project of the field of medicine and agriculture science from Shanghai Municipal Science and Technology Commission (15411951600); Shanghai science and technology commission foundation key project; Longhua Medical Project (LYTD-25); Ministry of Education Returned Scientific Research Foundation; Trans-Century Training Programme Foundation for the Talents by the State Education Commission (to J Zhang).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- Daly JM, Fry WA, Little AG, et al. Esophageal cancer: Results of an American College of Surgeons patient care evaluation study. J Am Coll Surg 2000;190:562-72; discussion 572-3. [PubMed]
- Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol 2009;24:729-35. [PubMed]
- van Loon AJ, Brug J, Goldbohm RA, et al. Differences in Cancer Incidence and Mortality among Socioeconomic Groups. Scand J Soc Med 1995;23:110-20. [PubMed]
- Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst 2002;94:334-57. [PubMed]
- Ayanian JZ, Zaslavsky AM, Fuchs CS, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol 2003;21:1293-300. [PubMed]
- Cronin-Fenton DP, Sharp L, Carsin AE, et al. Patterns of care and effects on mortality for cancers of the oesophagus and gastric cardia: A population-based study. Eur J Cancer 2007;43:565-75. [PubMed]
- von Bueren AO, Shalaby T, Oehler-Jänne C, et al. RNA interference-mediated c-MYC inhibition prevents cell growth and decreases sensitivity to radio- and chemotherapy in childhood medulloblastoma cells. BMC Cancer 2009;9:10. [PubMed]
- O’Malley CD, Le GM, Glaser SL, et al. Socioeconomic status and breast carcinoma survival in four racial/ethnic groups-A population-based study. Cancer 2003;97:1303-11. [PubMed]
- Wang LD, Zhou FY, Li XM, et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet 2010;42:759-U46. [PubMed]
- Das A, Singh V, Fleischer DE, et al. A comparison of endoscopic treatment and surgery in early esophageal cancer: An analysis of surveillance epidemiology and end results data. Am J Gastroenterol 2008;103:1340-5. [PubMed]
- Zhang J, Chen SF, Zhen Y, et al. Multicenter Analysis of Lung Cancer Patients Younger Than 45 Years in Shanghai. Cancer 2010;116:3656-62. [PubMed]
- Bao PP, Zheng Y, Wang CF, et al. Time Trends and Characteristics of Childhood Cancer Among Children Age 0-14 in Shanghai. Pediatr Blood Cancer 2009;53:13-6. [PubMed]
- Greenstein AJ, Litle VR, Swanson SJ, et al. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer 2008;112:1239-46. [PubMed]
- Esophageal cancer: epidemiology, pathogenesis and prevention. Nat Clin Pract Gastroenterol Hepatol 2008;5:517-26. [PubMed]
- Guohong Z, Min S, Duenmei W, et al. Genetic heterogeneity of oesophageal cancer in high-incidence areas of southern and northern China. PLoS One 2010;5:e9668. [PubMed]
- Zhang W, Bailey-Wilson JE, Li W, et al. Segregation analysis of esophageal cancer in a moderately high-incidence area of northern China. Am J Hum Genet 2000;67:110-9. [PubMed]
- Cui R, Kamatani Y, Takahashi A, et al. Functional Variants in ADH1B and ALDH2 Coupled With Alcohol and Smoking Synergistically Enhance Esophageal Cancer Risk. Gastroenterology 2009;137:1768-75. [PubMed]
- Tran GD, Sun XD, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian General Population Trial cohort in China. Int J Cancer 2005;113:456-63. [PubMed]
- Chow WH, Blot WJ, Vaughn TL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst 1998;90:150-5. [PubMed]
- Siassi F, Ghadirian P. Riboflavin deficiency and esophageal cancer: A case control-household study in the Caspian Littoral of Iran. Cancer Detect Prev 2005;29:464-9. [PubMed]
- Wang J, Wang S, Su J, et al. Food contamination of fumonisin B1 in high-risk area of esophageal and liver cancer. Toxicol Sci 2003;72:188. [PubMed]
- Steyerberg EW, Neville B, Weeks JC, et al. Referral patterns, treatment choices, and outcomes in locoregional esophageal cancer: A population-based analysis of elderly patients. J Clin Oncol 2007;25:2389-96. [PubMed]
- Greenstein AJ, Litle VR, Swanson SJ, et al. Prognostic significance of the number of lymph node metastases in esophageal cancer. J Am Coll Surg 2008;206:239-46. [PubMed]


