
Enhanced recovery after surgery (ERAS®) protocol adapted to the Brazilian reality: a prospective cohort study for thoracic patients
Introduction
The Enhanced Recovery After Surgery (ERAS®) Protocol aims to improve surgical patients’ outcomes by implementing a systematic and multidisciplinary plan of care (1), which encompass the three phases of perioperative care: Preoperative, intraoperative, and postoperative (2). The protocol was initially developed for colorectal surgery, and since then, it has been continuously spreading to other surgical specialties (3), such as thoracic surgery (4-13).
Nonetheless designing and implementing protocols can be a challenge. The scenario in Low-Middle income countries (LMICs) demands tailored protocols that are capable of associating local comorbidities, infrastructure, professionals and other resource availability (14,15). Specifically, in Latin America, a pioneering multimodal program was initiated in Brazilian territory by 2005 (16). This project, titled ACERTO, organizes seminars and courses for assisting on the dissemination of ERAS® concepts (16,17). For this reason, subsequently years were followed by multiple Brazilian hospitals achieving ERAS® accreditation (17).
Published Brazilian literature demonstrated the benefits of ERAS protocol applied to hepatic resections (18) and bariatric (19) patients. Despite LMICs research is progressing, further work is needed to identify capacity (human, material and networking) within effective strategies (20). To the best of our knowledge, this is the first Brazilian prospective study (in the setting of thoracic surgery) that provides some clinical insight by describing, analyzing and comparing the impacts of PROSM (Protocolo de Recuperação Cirúrgica Acelerada Santa Marcelina—Santa Marcelina’s Enhanced Protocol) (21), a designed tool based on ERAS® recommendations and tailored to meet hospital (located in the city of Sao Paulo, Brazil) and the patients’ needs. This is a preliminary analysis of the PROSM protocol applicability before the randomized trial (Clinical Trials.gov - NCT03271749), with an independent sample between the randomized trial and this study.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-920) (22).
Methods
Patient selection and data collection
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institution research ethics committee’s (Number 70142917.0.0000.0066 – Version 4, August 2, 2019).
Consecutive PROSM patients’ data were prospectively collected and organized for analysis. The control group comprised patients who were retrospectively selected before PROSM adoption, matching the intervention group by surgical indication. The group of surgeons maintains a database of some patients who have undergone thoracic surgery and provided their informed consent to store and use data for research purposes. For this research, we extracted patients from the same institution. Due to the impaired availability of literature directed to LMICs in the thoracic segment (4-10), our analysis has a pilot study character. Thus, a sample size estimation was not performed. Propensity matching score was not estimated since this study was considered a pilot for the clinical trial (Clinical Trials.gov - NCT03271749). Likewise, the research team did not choose to have a prospective study with patients that were not submitted to PROSM because since PROSM implementation in 2017, all thoracic surgical patients were submitted to our protocol.
Included patients were ≥18 years old at the time of the surgical intervention (performed between April 30th 2014 and December 28th 2016 for the control patients, and December 13th, 2017, and July 29th 2019 for PROSM patients), and with elective indications for pulmonary resections (mediastinum, biopsies, pulmonary resections for benign and malignant conditions or metastasis). All patients included in the study signed the informed consent. We did not start the prospective study until the protocol was fully integrated in the institution.
Patients unable to provide the informed consent form, compromised performance status (ECOG >2), body weight <19 kg/m2 or >31 kg/m2, history of allergies to any of the drugs used in the anesthesia for PROSM or latex, renal dysfunction, liver dysfunction (Child B and C) or Heart Failure (functional classes III and IV) were excluded. Patients who did not emerge from the anesthesia, unable to maintain the level of consciousness (to understand and respond to verbal commands) or presented orthostatic hypotension during anesthesia awakening, were also excluded from this study.
In attempt to control bias induced by multiple surgical techniques, all surgery (open or performed by thoracoscopy) was performed by one of the three surgeons of the surgical group (rotation schedule) and one surgical technician. Likewise, for PROSM patients, a pre-established anesthetic protocol was adopted. Rapid metabolism drugs were chosen and adjusted to the patient’s body mass and according to the bispectral index (BIS) analysis. Propofol was used to induce hypnosis, and remifentanil to maintain intraoperative analgesia. Regional anesthesia was performed by a paravertebral blockade, following the spinal erector muscle topography on the operated side. The blockade was composed by multi-drug analgesic solution called PTAS, which consists of: 1 mcg/kg of clonidine, 5 mg of ketamine, 7.5 mg of ropivacaine, 10 mg of lidocaine, 10 mg of dexamethasone, 500 mg of hydrocortisone, 20 µL of 8.4% sodium bicarbonate solution and 1,000 mg of magnesium sulfate. All drugs were diluted in 500 mL of 0.9% saline solution. Epidural catheter was not used. For the control group, analgesia plan was at surgeon’s discretion.
Data were collected and organized in Microsoft Excel® spreadsheets. The following variables were extracted from all patients: gender, age, height, weight, diagnosis, surgical procedures, ICU admission, clinical complications, surgical complications, reoperation, need of thoracentesis, ICU and hospital length of stay (LOS), thoracic drain duration and costs [materials, surgical procedure, daily (medication, and infrastructure), total and post-surgical costs (comprised ICU and hospital costs after surgery)]. The post-surgical cost was calculated after patient’s discharge, based on the costs per day after the surgical intervention We did not encounter loss of data.
Material costs were expenditures related to materials that were used during the surgical intervention and post-operatory period, including medication, instruments, and oxygen. Surgical costs were costs related to materials used during intervention and surgical theater allocation.
Patients were admitted in the ICU if they presented cardiological or cerebrovascular comorbidities or if they were submitted to lobectomy or mediastinal tumor resection. Criteria for a chest tube removal was the absence of air leaks or that drained less than 200 mL of fluid. Both criteria were used in the intervention and control groups.
PROSM protocol development
The PROSM protocol is an adapted version of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS) (1) recommendations. Tables S1-S3 describe the adapted protocol phases (Preoperative, Intraoperative and Postoperative) comparing with ERAS® recommendations.
Statistical analysis
Statistical analysis was performed using SPSS (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp). Associations between continuous variables were assessed using the nonparametric Mann-Whitney U test for non-normally distributed data (ICU LOS, hospital LOS, drain duration, cost analysis), and Student’s t test was used for normally distributed data [Age and Body Mass Index (BMI)]. Categorical measures (gender, surgical complications, clinical complications, Intensive Care Unit (ICU) admission, reoperation, and mortality) were analyzed using χ2 or Fisher’s exact test. The likelihood ratio was used in cases that χ2 presented violated assumptions. Normally distributed data were described as mean and standard deviations (SD). Non-normally distributed data were described as median and interquartile range (IQR). ICU and Hospital LOS were also reported as mean only to demonstrate variability. A subgroup analysis was performed without ICU patients, to better analyze the impact of PROSM on overall costs, since ICU considerably increases overall costs. Welch’s t-test was used for normally distributed data, due to unequal sample sizes and/or unequal variances. Fisher’s exact test was applied for diagnosis and surgical intervention. All other variables followed the previously mentioned statistical tests. Spearman’s rho correlation (rs) was tested to identify the association between PROSM protocol, Hospital LOS, ICU LOS and drain duration and Post-Surgical Costs. The correlation coefficient interpretation was (23): perfect (±1), very strong (±0.8 to ±0.9), moderate (±0.6 to ±0.7), fair (±0.3 to ±0.5) and poor (less than ± 0.3). Statistical significance was established at P<0.05.
Results
This sample was comprised by 122 participants. Patients’ baseline characteristics are demonstrated in Table 1. The total number of patients in the PROSM group was 61 patients (25 men and 36 women) and 61 patients in the Control group (19 men and 42 women). Mean age was 51.4±17.09 years and 51.3±17.23 years, and mean BMI was 25.8±3.64 kg/m2 and 25.7±4.86 kg/m2 in the PROSM and Control groups, respectively. There was no statistical significance regarding the patients’ baseline characteristics.
Table 1
| Variable | PROSM group (n=61) | Control group (n=61) | P value |
|---|---|---|---|
| Age, years; mean (SD) | 54.1 (17.09) | 51.3 (17.23) | 0.369a |
| Gender, n (%) | |||
| Male/Female | 25 (41.0)/36 (59.0) | 19 (31.1)/42 (68.9) | 0.258b |
| BMI, kg/m2; mean (SD) | 25.8 (3.64) | 25.7 (4.86) | 0.884a |
| VATS, n (%) | 11 (18.0) | 8 (13.1) | 0.454a |
| Principal surgery [n (%)] and diagnosis (n) | 1.000c | ||
| Bullectomy | 2 (3.3) | 2 (3.3) | |
| Tumor–Benign | 2 | 2 | |
| Lobectomy | 9 (14.8) | 9 (14.8) | |
| Tumor–Benign | 1 | 1 | |
| Tumor–Malignant | 8 | 8 | |
| Pneumectomy | 2 (3.3) | 2 (3.3) | |
| Tumor–Malignant | 2 | 2 | |
| Cist resection | 1 (1.6) | 1 (1.6) | |
| Mediastinum | 1 | 1 | |
| Mediastinal tumor resection | 4 (6.6) | 4 (6.6) | |
| Segmentectomy | 40 (65.5) | 40 (65.5) | |
| Metastasis | 14 | 14 | |
| Biopsies | 19 | 19 | |
| Tumor–Benign | 7 | 7 | |
| Thymectomy | 3 (4.9) | 3 (4.9) | |
a, Student’s
All patients were matched for surgical procedures and diagnosis between the groups. Both groups presented similar clinical complications, surgical complications, and reoperation rates (PROSM group: 1 thoracic wall hematoma, 1 hemothorax and 1 pleural effusion; Control group: 1 Hemothorax) and mortality (Control Group: Pneumonia – 1 patient). None of the patients needed thoracentesis, and none of the reoperation causes were due to early chest removal in PROSM patients.
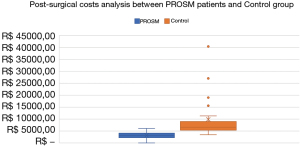
Clinical outcomes are displayed in Table 2. Statistical significance was found on ICU LOS (mean of 0.3±0.58 versus 1.2±1.65 days, P=0.001), Hospital LOS (mean of 1.6±1.32 versus 3.9±3.25 days, P=0.001) and Chest Drain duration (Median 1.0±1.00 versus 3.0±3.00 days, P=0.001) in the PROSM and Control groups, respectively. All costs found statistical significance apart from Materials and Medication (PROSM group: Median R$ 2,458.26, IQR R$ 3,536.30; Control group: Median R$ 2,052.35, IQR R$ 4,319.70, P=0.933). Figure 1 demonstrate the post-surgical cost analysis between the PROSM and control.
Table 2
| Variable | PROSM group (n=61) | Control group (n=61) | p value |
|---|---|---|---|
| ICU admission, n (%) | 14 (23.0) | 28 (45.9) | 0.008a |
| Clinical complications, n (%) | 5 (8.2) | 6 (9.8) | 0.752a |
| Surgical complications, n (%) | 4 (6.6) | 9 (14.8) | 0.142a |
| Reoperation, n (%) | 3 (4.9) | 1 (1.6) | 0.619b |
| Mortality, n (%) | 0 (0.0) | 1 (1.6) | 1.000b |
| ICU LOS, days | |||
| Median (IQR) | 0.0 (0.00) | 0.0 (2.00) | 0.001c |
| Mean (SD) | 0.3 (0.58) | 1.2 (1.65) | |
| Hospital LOS, days | |||
| Median (IQR) | 1.0 (1.00) | 3.0 (3.00) | <0.001c |
| Mean (SD) | 1.6 (1.32) | 3.9 (3.25) | |
| Chest drain duration, days | <0.001c | ||
| Median (IQR) | 1.0 (1.00) | 3.0 (3.00) | |
| Matmed costs, R$ | 2,458.26 | 2,052.35 | 0.933c |
| Median (IQR) | (3,536.30) | (4,319.70) | |
| Procedure costs, R$ | 2,726.47 | 4,311.41 | <0.001c |
| Median (IQR) | (1,709.58) | (2,205.63) | |
| Daily costs, R$ | 2,627.78 | 4,848.36 | <0.001c |
| Median (IQR) | (1,780.15) | (4,327.82) | |
| Total costs, R$ | 8,119.15 | 10,998.36 | <0.001c |
| Median (IQR) | (5,577.93) | (9,763.48) | |
| Post-surgical costs, R$ | 4,068.92 | 6,626.96 | <0.001c |
| Median (IQR) | (4,068.92) | (6,626.96) |
a, Chi-Square test; b, Fisher’s exact test; c, Mann-Whitney-U test. PROSM, Santa Marcelina’s Enhanced Protocol; ICU, Intensive Care Unit; LOS, length of stay; MatMed, materials and medication.
Tables S4,S5 display the full understanding of Spearman’s correlation. All variables were statistically significant. Strong correlation was present between chest tube duration and Hospital LOS (rs=0.88). Moderate correlation was present between Hospital LOS and PROSM (rs=0.60) and between Hospital LOS and Post-Surgical Costs (rs=0.62). PROSM demonstrated a poor correlation between Surgical and Clinical complications (rs=−0.13 and rs=−0.03, respectively).
Our group decided to include a subgroup analysis of results excluding patients that were admitted to the ICU after the surgical intervention (which would imply in higher overall costs). This analysis resulted in 47 (22 men, 25 women) PROSM participants and 33 (8 men and 25 women) Control participants.
The PROSM group was statistically significant for Hospital LOS (PROSM group median 1, IQR 1, Control group median 2, IQR 1, days, P<0.001), chest tube duration (PROSM group median 1, IQR 1, Control group median 2, IQR 1, days, P<0.001), Procedure Costs (PROSM group median R$ 2,412.07, IQR R$ 1,206.42, Control group median R$ 3,566.94, IQR R$ 5,213.38, P<0.001) and daily Costs (PROSM group median R$ 2,271.74, IQR R$ 1,102.93, Control group median R$ 3,274.92, IQR R$ 1,638.17, P=0.007). Patients’ demographics and outcomes are displayed in Tables S6,S7.
Discussion
The PROSM showed an overall compliance with several strong recommendations provided by ERAS® Protocol. We also included three extra domains (postoperative roentgenogram, postoperative laboratory tests and discharge guidance) and adapted to fulfil the context of local resources (Tables S1-S3). As suggested by literature (24), we did not modify strong recommendations and we applied this protocol as a self-assessment instrument, aiming to improve the quality of the delivered care and management of hospital resources.
As for the primary analysis (results that were directly associated with the patient), baseline characteristics, such as age, gender, and BMI (Table 1) did not result in statistical significance, suggesting homogeneity between the samples. Clinical and surgical complications (Table 2) were similar between the intervention and control groups. Analogous observations were also reported by previous studies (11-13), encouraging PROSM safety trend of not increasing complication rates.
Furthermore, our samples did not demonstrate statistically significant differences between patients that were or were not submitted to video-assisted thoracoscopic surgery (VATS) between groups (Table 1), which did not contribute as a source of bias due to the intrinsic benefit of a less invasive procedure that is associated with a reduced adverse event rate (25,26). Enhanced protocol patients that were submitted to VATS do not usually benefit as much as conventional surgery patients did (27).
The clinical aggravation of one patient in the control group, caused by pneumonia, was classified as a major complication, leading to the patient’s death. Additionally, four patients experienced major complications that lead to reoperation, with no further events. Despite the earlier removal of thoracic tubes observed in the PROSM group [PROSM: median 1.0 days (IQR 1.00), Control: median 3.0 days (IQR 3.00), P<0.001], no statistical significance was found between groups for clinical and surgical complications. Likewise, no correlation was found between PROSM and surgical/clinical complications.
Previous studies (12,27) demonstrated a significant decreased rate of pulmonary complications in the enhanced protocol samples. Our result dissimilarly could be explained by our modest sample of patients.
Nevertheless, PROSM can suggest clinical significance as the decreased exposure to the nosocomial environment, since PROSM patients had lower ICU LOS [PROSM: median 0.0 days (IQR 0.00); Control: median 0 day (IQR 2.00), P=0.001], Hospital LOS [PROSM: median 1.0 days (IQR 1.00); Control: median 3.0 days (IQR 3.00), P<0.001] and chest drain duration. Similar protocols also demonstrated decreased ICU and hospital LOS and chest drain duration (11,28,29) in the intervention group.
Contradictorily, one randomized trial (12) reported no difference in ICU and hospital LOS and drain duration. However, some aspects are worth being mentioned. The median hospital LOS resulted in 11 days for the control and intervention groups, which is significantly higher than other studies (8,13,30) and could reflect the institution strict protocols of prolonged preoperative preparation or later discharge. Likewise, the protocol did not specify chest drain management.
LOS reduction has been explained by reduced volume of sedation, early mobilization and later carbohydrate ingestion in preoperative prepare (8,31), as also adopted in PROSM. Remarkably, PROSM’s early mobilization is initiated within 2 hours after the surgical procedure: if patient is intubated, the physiotherapist assists upper limb mobilization. Once the patient is fully conscious, active, and able to answer commands, active physiotherapy is initiated with upper and lower limb mobility, until patients’ tolerance to orthostatic physiotherapy. The orthostatic position is held for 4 minutes; if no complications occurred (lipothymia, hypotension and nausea/vomiting), the patient is stimulated to walk with assistance. We believe that the early mobilization and pain control were important aspects that contributed to decrease ICU LOS.
Likewise, Das-Neves-Pereira et al. (32) concluded that the availability of a multidisciplinary team and family support is directly associated with the reduction of LOS in fast-track protocol for lung cancer lobectomies, although, Madani et al. (30) recognized that the entire protocol pathway is expected to be more important than single domains.
The entire cost summary was classified as a secondary analysis, demonstrating that PROSM contributed to more than R$ 500,000.00 in savings concerning overall and post-surgery costs among all the sample (37% of economy, per PROSM Patient). Except for materials and medication, all costs were statistically significant in the PROSM group (Procedure cost: 41%; Daily Cost: 15%; Total Cost: 64% of economy), which is also consistent with the literature (13,33,34).
Reducing in-hospital expenditures can assist health care institutions to better allocate resources, aiming an efficient and cost-effective patient management whilst maintaining the standard of care. Sammour et al. (35) analyzed the overall costs of ERAS in elective colonic surgery, which resulted in an overall cost saving of NZ$ 6900 per patient, including the implementation cost of NZ$ 102,000. A hospital in Virginia was able to admit 28.1 additional patients after being capable of lowering 5.5 days in the LOS, after the enhanced protocol implementation (13).
Murphy and Topel (36) developed an economic framework for assessing improvements in life expectancy and health. As a result, 1% of reduction in cancer mortality would be worth nearly 500 billion dollars, demonstrating how substantial improvement in technologies and protocols can assist cost management. Likewise, a Canadian group (37) verified that a lung resection enhanced protocol for lung resection assisted on saving CAD 4,396 by diminishing productivity loses.
In LMICs, a demand for resource optimization and infrastructure availability is recurrent (38,39). According to the United Nations (40), the global population could reach 8.5 billion people in 2030, demonstrating the importance to provide excellent care, control public and private expenditure burden and reestablishing patients’ and caregivers’ social responsibilities. Likewise, collaboration between LMICs and high-income countries can assist in optimizing knowledge for guidelines implementation (14,15). In our experience, PROSM can assist hospitals to improve their process and achieve a future ERAS Certification.
Likewise, the subgroup analysis supported the statistical significance of PROSM in Hospital LOS, drain duration, Procedure Costs and Post-Surgical costs, suggesting that PROSM also benefits patients who are not referred to the ICU. Surprisingly, Daily costs did not show statistical significance, which could suggest the influence of preoperative admission costs. Improvements can be done to optimize patient admission and preoperative preparation.
Our study has some limitations. Firstly, some bias could not be controlled due to the methodological design of a prospective study. Fiore et al. (41) had already raised the idea that there are a small number of non-randomized studies regarding this subject. The results should be interpreted with caution due to methodological flaws. We will be able to better control this bias once the randomized trial is completed. Secondly, we did not measure the compliance for each patient, making it difficult to measure changes that might occur over time. Thirdly, our results were obtained from a small sample that underwent surgical and diagnostic procedures, which can increase their heterogeneity.
Conclusions
PROSM patients experienced reduced ICU LOS, Hospital LOS and Chest Drain duration. Cost analysis, such as the procedure, daily, total and post-surgical costs were also favored the PROSM group.
To the best of our knowledge, this is the first study to present a detailed protocol and contemplate surgical outcomes and cost analysis of a thoracic enhance protocol adapted in a Brazilian reality. This study disclosed important aspects related to the improvement of quality of the delivered care and the opportunity for cost management, which are recurrent in LMIC. We expect to assist more countries to improve knowledge under the implementation of enhanced protocols.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-920
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-920
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-920). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee board of 70142917.0.0000.0066 – Version 4, August 2, 2019). All patients included in the study signed the informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Van Haren RM, Atay SM. Enhancing the study of enhanced recovery after thoracic surgery: methodology and population-based approaches for the future. J Thorac Dis 2019;11:S612-8. [Crossref] [PubMed]
- Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630-41. [Crossref] [PubMed]
- Semenkovich TR, Hudson JL, Subramanian M, et al. Enhanced Recovery After Surgery (ERAS) in Thoracic Surgery. Semin Thorac Cardiovasc Surg 2018;30:342-9. [Crossref] [PubMed]
- Batchelor TJP, Ljungqvist O. A surgical perspective of ERAS guidelines in thoracic surgery. Curr Opin Anaesthesiol 2019;32:17-22. [Crossref] [PubMed]
- Medbery RL, Fernandez FG, Khullar OV. ERAS and patient reported outcomes in thoracic surgery: a review of current data. J Thorac Dis 2019;11:S976-86. [Crossref] [PubMed]
- Khandhar SJ, Schatz CL, Collins DT, et al. Thoracic enhanced recovery with ambulation after surgery: a 6-year experience. Eur J Cardiothorac Surg 2018;53:1192-8. [Crossref] [PubMed]
- Rogers LJ, Bleetman D, Messenger DE, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg 2018;155:1843-52. [Crossref] [PubMed]
- Brunelli A, Thomas C, Dinesh P, et al. Enhanced recovery pathway versus standard care in patients undergoing video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2017;154:2084-90. [Crossref] [PubMed]
- Giménez-Milà M, Klein AA, Martinez G. Design and implementation of an enhanced recovery program in thoracic surgery. J Thorac Dis 2016;8:S37-45. [PubMed]
- Salati M, Brunelli A, Xiumè F, et al. Does fast-tracking increase the readmission rate after pulmonary resection? A case-matched study. Eur J Cardiothorac Surg 2012;41:1083-7; discussion 1087. [Crossref] [PubMed]
- Muehling BM, Halter GL, Schelzig H, et al. Reduction of postoperative pulmonary complications after lung surgery using a fast track clinical pathway. Eur J Cardiothorac Surg 2008;34:174-80. [Crossref] [PubMed]
- Martin LW, Sarosiek BM, Harrison MA, et al. Implementing a Thoracic Enhanced Recovery Program: Lessons Learned in the First Year. Ann Thorac Surg 2018;105:1597-604. [Crossref] [PubMed]
- Tabyshova A, Hurst JR, Soriano JB, et al. Gaps in COPD Guidelines of Low- and Middle-Income Countries: A Systematic Scoping Review. Chest 2021;159:575-84. [Crossref] [PubMed]
- Olayemi E, Asare EV, Benneh-Akwasi Kuma AA. Guidelines in lower-middle income countries. Br J Haematol 2017;177:846-54. [Crossref] [PubMed]
- de-Aguilar-Nascimento JE, Salomão AB, Waitzberg DL, et al. ACERTO guidelines of perioperative nutritional interventions in elective general surgery. Rev Col Bras Cir 2017;44:633-48. [Crossref] [PubMed]
- Loughlin SM, Alvarez A, Falcão LFDR, et al. The History of ERAS (Enhanced Recovery After Surgery) Society and its development in Latin America. Rev Col Bras Cir 2020;47:e20202525 [Crossref] [PubMed]
- Teixeira UF, Goldoni MB, Waechter FL, et al. ENHANCED RECOVERY (ERAS) AFTER LIVER SURGERY:COMPARATIVE STUDY IN A BRAZILIAN TERCIARY CENTER. Arq Bras Cir Dig 2019;32:e1424 [Crossref] [PubMed]
- Gouveia de Oliveira MP, Fernandes G, Andrade JF, et al. Impact of Enhanced Recovery After Bariatric Surgery (ERABS) Protocol in Reducing Length of Stay and Hospitalization Costs: the Experience of a Philanthropic Hospital in Brazil. Obes Surg 2021;31:1612-7. [Crossref] [PubMed]
- Franzen SR, Chandler C, Lang T. Health research capacity development in low and middle income countries: reality or rhetoric? A systematic meta-narrative review of the qualitative literature. BMJ Open 2017;7:e012332 [Crossref] [PubMed]
- de Abreu IRLB, Abrão FC, Cavalcante MGC, et al. Clinical trial report: protocol for accelerated recovery in patients undergoing thoracic surgical procedures (PROSM). Study randomized comparative between the adoption of the proposed guidelines and the traditional method currently used in the institution. EC Pulmonol Respir Med 2020;9:24-9.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573-7. [Crossref] [PubMed]
- Chan YH. Biostatistics 104: correlational analysis. Singapore Med J 2003;44:614-9. [PubMed]
- Woolf SH, Grol R, Hutchinson A, et al. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ 1999;318:527-30. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23. [PubMed]
- Boffa DJ, Dhamija A, Kosinski AS, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg 2014;148:637-43. [Crossref] [PubMed]
- Van Haren RM, Mehran RJ, Mena GE, et al. Enhanced Recovery Decreases Pulmonary and Cardiac Complications After Thoracotomy for Lung Cancer. Ann Thorac Surg 2018;106:272-9. [Crossref] [PubMed]
- Scarci M, Solli P, Bedetti B. Enhanced recovery pathway for thoracic surgery in the UK. J Thorac Dis 2016;8:S78-83. [PubMed]
- Zhao G, Huang Y, Chen X, et al. Research on fast track surgery application in lung cancer surgery. Zhongguo Fei Ai Za Zhi 2010;13:102-6. [PubMed]
- Madani A, Fiore JF Jr, Wang Y, et al. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery 2015;158:899-908; discussion 908-10. [Crossref] [PubMed]
- ERAS Compliance Group. The Impact of Enhanced Recovery Protocol Compliance on Elective Colorectal Cancer Resection: Results From an International Registry. Ann Surg 2015;261:1153-9. [Crossref] [PubMed]
- Das-Neves-Pereira JC, Bagan P, Coimbra-Israel AP, et al. Fast-track rehabilitation for lung cancer lobectomy: a five-year experience. Eur J Cardiothorac Surg 2009;36:383-91; discussion 391-2. [Crossref] [PubMed]
- Maruyama R, Miyake T, Kojo M, et al. Establishment of a clinical pathway as an effective tool to reduce hospitalization and charges after video-assisted thoracoscopic pulmonary resection. Jpn J Thorac Cardiovasc Surg 2006;54:387-90. [Crossref] [PubMed]
- Zehr KJ, Dawson PB, Yang SC, et al. Standardized clinical care pathways for major thoracic cases reduce hospital costs. Ann Thorac Surg 1998;66:914-9. [Crossref] [PubMed]
- Sammour T, Zargar-Shoshtari K, Bhat A, et al. A programme of Enhanced Recovery After Surgery (ERAS) is a cost-effective intervention in elective colonic surgery. N Z Med J 2010;123:61-70. [PubMed]
- Murphy KM, Topel RH. The Value of Health and Longevity. J Polit Econ 2006;114:871-904. [Crossref]
- Paci P, Madani A, Lee L, et al. Economic Impact of an Enhanced Recovery Pathway for Lung Resection. Ann Thorac Surg 2017;104:950-7. [Crossref] [PubMed]
- Russel L. Intensive Care. In: Technology in Hospitals: Medical Advances and Their Diffusion. Washignton: The Brookings Institute; 1979:42-9.
- Costa G, Thuler LC, Ferreira CG. Epidemiological changes in the histological subtypes of 35,018 non-small-cell lung cancer cases in Brazil. Lung Cancer 2016;97:66-72. [Crossref] [PubMed]
- United Nations. Population size, growth and age structure. In: World Population Prospects 2019 Highlights. New York, 2019:5-22. Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf
- Fiore JF Jr, Bejjani J, Conrad K, et al. Systematic review of the influence of enhanced recovery pathways in elective lung resection. J Thorac Cardiovasc Surg 2016;151:708-715.e6. [Crossref] [PubMed]


