
BRCA-associated protein 1 (BAP1) and miR-31 combination predicts outcomes in epithelioid malignant pleural mesothelioma
Introduction
Malignant pleural mesothelioma (MPM) is a rare and aggressive disease with a dismal prognosis (1). Overall survival (OS) usually ranges between 9 and 18 months. A multimodal approach including surgery, radiation therapy and systemic chemotherapy is reserved to a limited proportion of patients with early stage MPM (2). Standard treatment for most of patients affected by advanced disease is represented by platinum-based chemotherapy (3).
MPM is classified as epithelioid (e-MPM), sarcomatoid (s-MPM) or biphasic (b-MPM). Histological subtype represents a well-known prognostic factor in MPM. In particular, patients with e-MPM have better clinical outcome compared to the other ones (4,5). Anyway, heterogeneous outcomes can be observed within the same histological subgroup, thus highlighting the need of a deeper insight into MPM biology and into novel prognostic and predictive biomarkers to improve patient risk stratification (6,7).
BRCA-associated protein 1 (BAP1) is a deubiquitinating enzyme involved in several cellular processes such as apoptosis, DNA repair and cell growth control and its alterations have been detected in about 60% of MPM (8-11). Despite the BAP1 loss, detected by immunohistochemical (IHC) analysis was mostly associated with germline and somatic gene mutations, about the 25% of MPM with negative nuclear IHC staining of BAP1 were negative for BAP1 gene mutations (12-15). It was reported that the frequency of somatic BAP1 mutations in MM varies considerably (13). This discrepancy appeared related to methodological approaches used to detect genetic alterations: the MM is characterized by minute deletions, which are not detected by NGS or genomic hybridization array (16). Although methylation changes have not been associated with BAP1 expression (13), a post-transcriptional mechanisms may be involved (12). MiRNA are small noncoding RNAs that control gene expression at post-transcriptional level binding by 7–8 nucleotides the complementary ones in the 3'-untranslated regions of their targets. MiRNA may function as either oncogene or tumour suppressors depending on target genes and cancer type (17). It has been demonstrated in pre-clinical studies that miR-31 is a post-transcriptional regulator of BAP1 (18-20). In this retrospective study, the interaction between BAP1 expression and miR-31 levels was analysed in patients affected by advanced MPM and their prognostic role within different MPM histotype was evaluated.
We present the following article in accordance with the REMARK reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-555).
Methods
Study population
We retrospectively examined 55 consecutive patients with a histological diagnosis of MPM referred to our Institution between 2005 and 2017. Data on age, gender, smoking status, histology, stage, previous surgery, type of chemotherapy treatment were retrieved. Benign asbestos-related diseases, such as fibrosis, pleural plaques and asbestosis, were documented by chest high-resolution computed tomography (HRCT) analysis. Asbestos exposure data were also recorded during the interview and patients who were working in the asbestos-related industry were considered exposed. In order to evaluate the prognostic role of the BAP1 and miR-31, progression-free survival (PFS), evaluated by HRCT analysis, overall survival (OS), and annual follow-up period were collected for each patient. Inclusion criteria were: complete clinical data, the availability of BAP1 staining and the availability of tissue samples. Patients with previous or synchronous second malignancies were excluded from the study. Archival formalin-fixed paraffin-embedded (FFPE) tissues, collected at diagnosis before the start of treatment, were analysed.
Tumour resection was performed in patient eligible for surgery. All patients received first-line platinum-based chemotherapy in association with pemetrexed or pemetrexed in monotherapy.
All participants provided their informed consent to participate in the study. The study was carried out according to the Helsinki Declaration (as revised in 2013) and ethical approval to conduct this study was granted by the Ethical committee of the University Hospital of Marche, N. 51/DG 05/02/2009, Italy.
Immunohistochemical analysis
All selected MPM histological samples were reviewed by an experienced mesothelioma pathologist (F.B.) and divided into epithelioid, sarcomatoid (including desmoplastic) and biphasic MPM according to the 2015 World Health Organization (WHO) classification (21). To include the MPM sample in the b-MPM subgroup, the presence of both sarcomatoid and epithelioid components was required at least in the ten percent of the tumor. All samples were FFPE and a single 4-mm-thick paraffin section was cut from the sample with the greatest amount of tumor tissue for each patient. All sections were deparaffinized and rehydrated in graded concentrations of xylene and ethanol. Sections were coated with 1:50 mouse monoclonal BAP1 antibody (clone C4:sc-28383; Santa Cruz Biotechnology, USA) and incubated at 4 °C overnight. Then, automated IHC was performed on Omnis platform (Agilent, USA). BAP1 IHC status was considered as “positive/retained” if there was an unambiguous positive nuclear staining in any number of tumor cells, and “negative/loss” if the nuclear staining was absent in neoplastic cells. Tumor cells with cytoplasmic reactivity without a clear nuclear staining were considered negative. Non-neoplastic cells, such as vascular endothelium, fibroblasts or inflammatory cells, were considered as internal positive control.
MiR-31 assay
Total RNA was extracted from FFPE tissue samples (10–100 µg) using the RecoverAll™ Total Nucleic Acid Isolation Kit for FFPE (Thermo Fisher Scientific) according to the manufacturer’s instructions. The RNA concentration and purity were determined in the Nanodrop 1,000 spectrophotometer (Thermo Fisher Scientific). MiR-31 first-strand cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative RT-PCR (qPCR) was performed using the TaqMan Fast Advanced Master Mix (Applied Biosystems) with U6 as the housekeeping gene. The qPCR assays were carried out in triplicate using the Mastercycler EP Realplex instruments (Eppendorf). MiR-31 levels were considered as percentiles and then divided into quartiles, in order to quantify the miR-31 expression for each sample. Because the 25th and 50th percentile showed similar miR-31 expression, the respective interquartiles were used as reference and results of remaining samples were expressed as relative level using the ∆CT method (2−∆CT). MiR-31 expression was considered “high” if miR-31 levels were over the 75th percentile and “low” if miR-31 levels were below the 75th percentile.
Statistical analysis
Discrete data were expressed either as mean, standard deviation, minimal and maximal values (if normally distributed), or as median, quartile and range (if not-normally distributed). Categorical variables were reported as either fractions or percentage and compared by means of chi-squared method. Comparisons between and among groups were performed by two-tailed Student’s t-test and one-way analysis of variance (ANOVA) with Tukey post-hoc analysis, respectively. The OS and PFS were estimated using Kaplan-Meier method and log-rank method was used to assess difference between subgroups. The OS was defined as the temporal interval between the date of the first cycle of first-line chemotherapy and the date of death or censoring at the date of last follow-up of alive patients. PFS was considered as the time from the first cycle of first-line chemotherapy until clinical or instrumental disease progression or last follow-up. Multivariate Cox regression analysis was used to evaluate independent predictors of survival. Nonsignificant prognostic factors were excluded from the model using backward elimination. A P value <0.05 was considered statistically significant. No correction for multiple testing was performed. All the analyses were performed by using SPSS for Windows version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Demographic and clinicopathological characteristics of MPM patients
Our cohort consists of 55 patients (84% male). Median age at diagnosis was 71 years (range, 52–81 years). The median OS was 14.6 (95% CI: 10.7–18.5) months and median PFS was 5.5 (95% CI: 3.1–7.9) months. Exposure to asbestos was documented in 47% of patients, and 65% of patients were current/former smokers. E-MPM represented 74% of cases, while 13% was represented by s-MPM and 13% was b-MPM. The 10% of patients showed distant metastases at diagnosis. Only 16% of patients underwent surgical resection and presented local recurrence or distant metastases afterwards. All patients received first-line platinum-based chemotherapy in association with pemetrexed (80%) or pemetrexed in monotherapy (20%). Demographic and clinic-pathological characteristics are summarized in Table 1.
Table 1
| Variable | BAP1 | Total (n=55) | P value | |
|---|---|---|---|---|
| Loss (n=33) | Retained (n=22) | |||
| Age (years) | 71.8±5.8 | 68.9±6.6 | 70.6±6.2 | 0.088 |
| Gender | 0.478 | |||
| Male | 27 (82%) | 19 (86%) | 46 (84%) | |
| Female | 6 (18%) | 3 (14%) | 9 (16%) | |
| Smoking | 0.411 | |||
| Yes | 22 (67%) | 14 (64%) | 36 (65%) | |
| No | 11 (33%) | 8 (36%) | 19 (35%) | |
| Asbestos exposure | 0.310 | |||
| Yes | 17 (52%) | 9 (41%) | 26 (47%) | |
| No | 16 (48%) | 13 (59%) | 29 (53%) | |
| Istotype | 0.016 | |||
| Epithelioid | 29 (88%) | 12 (55%) | 41 (74%) | |
| Sarcomatoid | 1 (3%) | 6 (27%) | 7 (13%) | |
| Biphasic | 3 (9%) | 4 (18%) | 7 (13%) | |
| Stage | 0.317 | |||
| Stage I/II | 3 (9%) | 3 (14%) | 6 (10%) | |
| Stage III/IV | 30 (91) | 19 (86%) | 49 (90%) | |
| miR-31 | 6.6±11.6 | 25.9±36.6 | 14.0±27.0 | 0.013 |
| Surgery | 0.478 | |||
| Yes | 6 (18%) | 3 (14%) | 9 (16%) | |
| No | 27 (82%) | 19 (86%) | 46 (84%) | |
| Chemotherapy | 0.602 | |||
| Pemetrexed | 7 (21%) | 4 (18%) | 11 (20%) | |
| Pemetrexed-Platinum | 26 (79%) | 18 (82%) | 44 (80%) | |
| OS (median months) | 14.9 [7.7–22.1] | 11.3 [4.9–17.6] | 14.6 [10.7–18.5] | 0.021 |
| PFS (median months) | 3.8 [0.0–8.1] | 5.5 [1.7–9.3] | 5.5 [3.1–7.9] | 0.243 |
BAP1, BRCA-associated protein 1; OS, overall survival; PFS, progression free survival.
Distribution of BAP1 and miR-31 in MPM patients and association with survival in the whole cohort
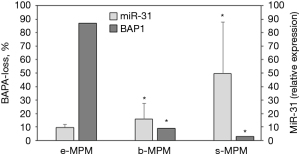
The BAP1 detected by IHC is shown in Figure 1. BAP1 loss was found in 60% of cases and was mainly associated with the epithelioid subtype (P=0.016). At univariate analysis, including all MPM patients, epithelioid subtype was associated with significant better OS [median OS for e-MPM was 18.4 (95% CI: 14.1–22.7) months vs. 8.6 (95% CI: 7.5–9.6) months for b-MPM and 6.0 (95% CI: 3.6–8.4) months for s-MPM, P=0.0005], but not with better PFS [median PFS for e-MPM was 7.7 (95% CI: 4.6–10.8) vs. 3.8 (95% CI: 2.5–5.1) months for b-MPM and 3.1 (95% CI: 0.0–6.2) for s-MPM, P=0.116]. As shown in Figure 2, BAP1 loss was significantly associated with longer OS [median OS was 14.9 (95% CI: 7.7–22.1) months for BAP1 loss vs. 11.3 (95% CI: 4.9–17.6) months for BAP1 retained, P=0.021], but not with longer PFS [median PFS was 3.8 (95% CI: 0.0–8.1) months for BAP1 loss vs. 5.5 (95% CI: 1.7–9.3) months for BAP1 retained, P=0.243]. The association of BAP1 loss with improved OS found in the univariate analysis was not confirmed in the multivariate analysis. At multivariate analysis, considering age, gender, smoking status, asbestos exposure, surgery, histotype and BAP1 status, only asbestos exposure and histotype resulted independent prognostic factors associated with OS (for epithelioid histotype, HR =0.156, 95% CI: 0.052–0.464, P=0.001; for asbestos exposure, HR 1.953, 95% CI: 1.006–3.789, P=0.048). None of the prognostic variables evaluated had a significant impact on PFS (Table 2). In the whole cohort, no difference in OS was observed in patients with low miR-31 levels compared to the ones with high miR-31 levels (P=0.124). Patients with BAP1 loss showed lower miR-31 levels compared to BAP1 retained MPM (P=0.013, Table 1). Epithelioid MPM subtype showed lower expression of miR-31 compared to the non-epithelioid MPM (P=0.04), and the low miR-31 levels were associated with the high percentage of MPM patients with BAP1 loss (Figure 3).
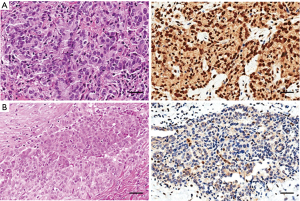
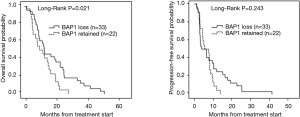
Table 2
| Variable | OS | PFS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI (HR) | P value | HR | 95% CI (OR) | P value | ||
| MPM (n=55) | |||||||
| Age | 0.977 | 0.917–1.041 | 0.470 | 0.971 | 0.911–1.036 | 0.370 | |
| Gender | 0.720 | 0.257–2.016 | 0.531 | 1.056 | 0.358–3.117 | 0.921 | |
| Smoking | 0.690 | 0.343–1.389 | 0.298 | 0.819 | 0.403–1.661 | 0.579 | |
| Asb-exp | 1.953 | 1.006–3.789 | 0.048 | 1.532 | 0.801–2.930 | 0.197 | |
| Surgery | 0.608 | 0.249–1.487 | 0.275 | 0.659 | 0.277–1.566 | 0.345 | |
| Histotype | 0.156 | 0.052–0.464 | 0.001 | 0.493 | 0.187–1.302 | 0.154 | |
| BAP1 | 0.747 | 0.347–1.608 | 0.456 | 0.889 | 0.425–1.858 | 0.754 | |
| Epithelioid MPM (n=40) | |||||||
| Age | 1.017 | 0.949–1.089 | 0.641 | 0.999 | 0.931–1.073 | 0.980 | |
| Gender | 0.731 | 0.198–2.695 | 0.638 | 1.377 | 0.319–5.954 | 0.668 | |
| Smoking | 0.605 | 0.246–1.483 | 0.272 | 0.753 | 0.311–1.823 | 0.529 | |
| Asb-exp | 2.082 | 1.082–4.004 | 0.028 | 1.513 | 0.745–3.072 | 0.252 | |
| Surgery | 0.420 | 0.173–1.021 | 0.056 | 0.424 | 0.156–1.152 | 0.093 | |
| BAP1-miR-31 | 2.207 | 1.062–4.587 | 0.034 | 2.146 | 1.050–4.388 | 0.036 | |
| Non-epithelioid MPM (n=13) | |||||||
| Age | 0.862 | 0.757–0.980 | 0.024 | 0.848 | 0.753–0.956 | 0.007 | |
| Gender | 0.266 | 0.017–4.235 | 0.348 | 0.434 | 0.022–8.401 | 0.581 | |
| Smoking | 0.426 | 0.103–1.756 | 0.238 | 0.297 | 0.057–1.541 | 0.148 | |
| Asb-exp | 8.163 | 0.876–76.06 | 0.065 | 4.673 | 1.096–19.91 | 0.037 | |
| Surgery | 7.367 | 0.661–82.04 | 0.104 | 1.130 | 0.095–13.42 | 0.923 | |
| BAP1-miR-31 | 0.714 | 0.117–4.360 | 0.715 | 6.430 | 1.350–30.62 | 0.019 | |
Regression model with stepwise Wald-backward adjusted for age, gender, smoking, surgery, histotype, and BAP1 or miR-31-BAP1. OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; MPM, malignant pleural mesothelioma; BAP1, BRCA-associated protein 1.
Prognostic value of BAP1 and miR-31 in epithelioid MPM patients
Considering the significant prognostic impact of epithelioid histotype on OS, we analysed the role of BAP1 status and miR-31 levels within the e-MPM subgroup. In this specific subtype, BAP1 alone was not able to significantly predict OS [median OS of 19.8 (95% CI: 6.3–33.3) months for BAP1 loss vs. 18.4 (95% CI: 15.6–21.2) months for BAP1 retained, P=0.271] and PFS [median PFS 6.3 (95% CI: 0.4–12.2) months for BAP1 loss vs. 8.1 (95% CI: 5.0–11.2) for BAP1 retained, P=0.453], (Figure 4A). Low miR-31 levels were significantly associated with better PFS [median 7.7 (95% CI: 2.95–12.4) months for low miR-31 levels vs. 5.9 (95% CI: 0.0–15.5) months for high miR-31 levels, P=0.028]. A trend, but not significant, toward better OS was also detected [median OS 18.4 (95% CI: 8.3–28.5) months for low miR-31 levels vs. 17.0 (95% CI: 10.9–23.1) months for high miR-31 levels, P=0.059], (Figure 4B). In addition, within the group of patients with highly expressed miR-31 (n=9), BAP1 retained was associated with better OS {median 23.2 [17–26] months for BAP1-retained vs. 11.3 [2–24] months for BAP1-loss, P=0.05} and PFS {median 8.1 [8–9] months for BAP1-retained vs. 2.0 [1–10] months for BAP1-loss, P=0.05}.
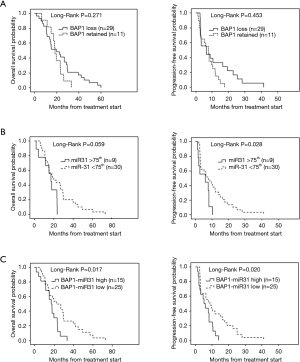
In order to further improve patient risk stratification, we stratified e-MPM patients according to BAP1 status and miR-31 levels taken together, obtaining a combined score (Figure 4C). Patients with BAP1 loss/low miR-31 levels (BAP1-miR-31 Low) had significantly better OS [median 22.6 (95% CI: 12.0–33.2) vs. 17.0 (95% CI: 11.5–22.5) months, P=0.017] and PFS [median 8.7 (95% CI: 3.3–14.1) vs. 5.1 (95% CI: 2.5–7.6) months, P=0.020], compared to the BAP1 retained/high miR-31 (BAP1-miR-31 High) subgroup. The multivariate analysis, showed in Table 2, confirmed BAP1 status/miR-31 level combination as an independent prognostic factor for e-MPM patients (HR of the BAP1 retained and high miR-31 level group 2.207, 95% CI: 1.062–4.587, P=0.034 for OS and HR 2.146, 95% CI: 1.050–4.388, P=0.036 for PFS). Conversely, patients with BAP1 loss/low miR-31 levels (BAP1-miR-31 Low) were associated with poor PFS in non-epithelioid MPM (HR 6.430, 95% CI: 1.350–30.62, P=0.019) along with age and asbestos exposure (Table 2).
Discussion
MPM is characterized by a very dismal prognosis and to date, platinum-based chemotherapy remains the standard treatment option for most patients. In daily clinical practice, heterogeneity of treatment response, also within the same MPM histotype, remains a hard challenge for clinicians. Therefore, identification of novel prognostic biomarkers may be helpful for patient risk stratification (22). BAP1 gene alterations, both inherited and somatic, have been detected in about 60% of MPM and their prognostic role has been amply investigated (23). Preclinical evidences suggest that BAP1 status might influence sensitivity of MPM cells to therapeutic drugs such as gemcitabine (24,25). Hassan et al. showed that OS following platinum-based chemotherapy was improved in patients affected by MPM with inherited mutations of several genes, such as BAP1, compared to patients without germline mutations (26). BAP1 has emerged as a critical tumor suppressor, predisposing to tumor development when mutated in the germline as well as somatically (13,14). The BAP1 mutation carriers affected the prognosis: MM arising in carriers of germline BAP1 mutations is clinically less aggressive and frequently associated with prolonged survival (26,27). Studies reported that inherited germline mutations influenced survival and helped identify relatives at risk for MM (28,29).
Since IHC on tumor tissue does not help to distinguish between somatic and germline mutations, the clinic relevance of somatic BAP1 loss-of-function in MPM remains under debate, with conflicting reports that it may be associated with epithelioid subtype (19,30). In accordance with our previous meta-analysis, the significant association of BAP1 loss with OS found in MPM was lost in multivariate analysis showing that the histological subtype mainly affected the patient survival (31). We also confirmed that BAP1 loss is more frequent in epithelioid histotype, as previously observed (32-34). Despite BAP1 loss has been associated with mutations or chromosomal deletions, BAP1 inactivation has been found also in tumors without BAP1 genetic alterations (12). Although this discrepancy has been mainly attributed to the methods used for genetic alteration detection, a post-transcriptional regulation mediated by miRNAs might be involved. MiR-31 has showed altered levels of expression in different tumors and it has been investigated as a potential upstream regulator of BAP1 (14,35-37). In MPM the role of miR-31 is still under investigation and data are conflicting. The MIR31HG gene, which encodes miR-31, lies adjacent to the CDKN2A/B locus, which is frequently deleted in MPM (38). Ivanov et al. reported that miR-31 loss in MPM cell lines was associated with cell cycle progression and its restoration inhibited cell proliferation and migration (38). Conversely, miR-31 over-expression in miR-31-null NCI-H2452 cells significantly increased resistance to cisplatin and carboplatin (39).
In the present preliminary study, we found that miR-31 was differentially expressed accordingly to the histotype: miR-31 was highly expressed in non-epithelioid MPM (b-MPM and s-MPM) compared to e-MPM. High miR-31 levels were also associated with BAP1 retained, worse PFS and a trend toward a shorter OS. In the cancerous tissue the direct relationship between miR-31 levels and BAP1 expression (high miR-31 corresponded to low BAP1 expression) previously described was lost (14,15). Probably, the genetic and epigenetic alterations in the malignant tissues contributed to a genetic rearrangement, thus affecting gene expression. Noteworthy, we did not perform a semiquantitative assay of BAP1 staining. Therefore, a weak effect of miR-31 on BAP1 protein levels cannot confidentially rule out.
By focusing on the e-MPM subgroup, high miR-31 levels correlated with worse PFS, and a trend to worse OS was also detected. Our results are consistent with data described by Matsumoto et al. showing a miR-31 upregulation in patients affected by s-MPM, which correlated with worse prognosis (40). Notably, the BAP1-miR-31 combination was strongly associated with OS and PFS, which was further confirmed in the multivariate model. Accordingly, retained BAP1 and high miR-31 expression was associated with a non-epithelioid and a more aggressive phenotype.
The anemia, as well the systemic immune–inflammation index (SII), has been described as powerful prognosis predicting factor in MPM (41). Here, by evaluating anemia and SII it was found that both biomarkers did not affect the OS and PFS either in univariate or multivariate analysis.
The MPM is a highly heterogeneous cancer characterized by multiple molecular profiles. Molecular diversity has been shown to occur between different histotype, as well as within specific histological subtypes. Therefore, we can postulate that the combination of BAP1 status with miR-31 levels may help to detect within the e-MPM an aggressive subtype with BAP1 retained and high miR-31 associated with a worse outcome.
Recently the EURACAN/IASLC (pathology) group published a study on “Updating the Histologic Classification of Pleural Mesothelioma”, underlining the importance of a multidisciplinary approach based on the integration of both histological and molecular parameters (42). More detailed diagnosis may lead to improved patients risk stratification, which is essential for guiding treatment. Alongside, a better knowledge of miRNAs role among different MPM histotype may lead to a better understanding of the complex MPM biology, as well as to the development of new miRNA-based targeted therapies.
Conclusions
The diagnostic criteria proposed for MPM in situ include a loss of BAP1 expression detected by IHC. In the last decades, the diagnostic utility of IHC has been integrated and enhanced by the development of methods including qPCR, able to investigate neoplasms at the molecular level. Despite being limited by the small sample size, the preliminary and retrospective nature of the study, we can postulate that both IHC and molecular approach are helpful in the context of e-MPM subtype in enhancing the diagnostic accuracy, thus providing further potential information with an impact on patient treatment. Prospective studies are needed to better analyze the role of this combined score in predicting outcome in MPM and explore the emerging idea of a molecular model classification complementary to the histological one, where miRNAs might play a key role.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-555
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-555
Peer Review File: Available at https://dx.doi.org/10.21037/jtd-21-555
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-555). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All participants provided their informed consent to participate in the study. The study was carried out according to the Helsinki Declaration and ethical approval to conduct this study was granted by the Ethical committee of the University Hospital of Marche, N. 51/DG 05/02/2009, Italy.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nicolini F, Bocchini M, Bronte G, et al. Malignant Pleural Mesothelioma: State-of-the-Art on Current Therapies and Promises for the Future. Front Oncol 2020;9:1519. [Crossref] [PubMed]
- Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1343-73. [Crossref] [PubMed]
- Katzman D, Sterman DH. Updates in the diagnosis and treatment of malignant pleural mesothelioma. Curr Opin Pulm Med 2018;24:319-26. [Crossref] [PubMed]
- Musk AW, Olsen N, Alfonso H, et al. Predicting survival in malignant mesothelioma. Eur Respir J 2011;38:1420-4. [Crossref] [PubMed]
- Brims FJ, Meniawy TM, Duffus I, et al. A Novel Clinical Prediction Model for Prognosis in Malignant Pleural Mesothelioma Using Decision Tree Analysis. J Thorac Oncol 2016;11:573-82. [Crossref] [PubMed]
- Zhou JG, Zhong H, Zhang J, et al. Development and Validation of a Prognostic Signature for Malignant Pleural Mesothelioma. Front Oncol 2019;9:78. [Crossref] [PubMed]
- Cantini L, Belderbos RA, Gooijer CJ, et al. Nivolumab in pre-treated malignant pleural mesothelioma: real-world data from the Dutch expanded access program. Transl Lung Cancer Res 2020;9:1169-79. [Crossref] [PubMed]
- Affar EB, Carbone M. BAP1 regulates different mechanisms of cell death. Cell Death Dis 2018;9:1151. [Crossref] [PubMed]
- Betti M, Aspesi A, Biasi A, et al. CDKN2A and BAP1 germline mutations predispose to melanoma and mesothelioma. Cancer Lett 2016;378:120-30. [Crossref] [PubMed]
- Alakus H, Yost SE, Woo B, et al. BAP1 mutation is a frequent somatic event in peritoneal malignant mesothelioma. J Transl Med 2015;13:122. [Crossref] [PubMed]
- Carbone M, Adusumilli PS, Alexander HR Jr, et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J Clin 2019;69:402-29. [Crossref] [PubMed]
- Betti M, Casalone E, Ferrante D, et al. Inference on germline BAP1 mutations and asbestos exposure from the analysis of familial and sporadic mesothelioma in a high-risk area. Genes Chromosomes Cancer 2015;54:51-62. [Crossref] [PubMed]
- Nasu M, Emi M, Pastorino S, et al. High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol 2015;10:565-76. [Crossref] [PubMed]
- Carbone M, Harbour JW, Brugarolas J, et al. Biological Mechanisms and Clinical Significance of BAP1 Mutations in Human Cancer. Cancer Discov 2020;10:1103-20. [Crossref] [PubMed]
- Murali R, Wiesner T, Scolyer RA. Tumours associated with BAP1 mutations. Pathology 2013;45:116-26. [Crossref] [PubMed]
- Yoshikawa Y, Emi M, Hashimoto-Tamaoki T, et al. High-density array-CGH with targeted NGS unmask multiple noncontiguous minute deletions on chromosome 3p21 in mesothelioma. Proc Natl Acad Sci U S A 2016;113:13432-7. [Crossref] [PubMed]
- Svoronos AA, Engelman DM, Slack FJ. OncomiR or Tumor Suppressor? The Duplicity of MicroRNAs in Cancer. Cancer Res 2016;76:3666-70. [Crossref] [PubMed]
- Yu M, Liang H, Fu Z, et al. BAP1 suppresses lung cancer progression and is inhibited by miR-31. Oncotarget 2016;7:13742-53. [Crossref] [PubMed]
- Wang N, Li Y, Zhou J. miR-31 Functions as an Oncomir Which Promotes Epithelial-Mesenchymal Transition via Regulating BAP1 in Cervical Cancer. Biomed Res Int 2017;2017:6361420 [Crossref] [PubMed]
- Sarcognato S, Gringeri E, Fassan M, et al. Prognostic role of BAP-1 and PBRM-1 expression in intrahepatic cholangiocarcinoma. Virchows Arch 2019;474:29-37. [Crossref] [PubMed]
- Galateau-Salle F, Churg A, Roggli V, et al. The 2015 World Health Organization Classification of Tumors of the Pleura: Advances since the 2004 Classification. J Thorac Oncol 2016;11:142-54. [Crossref] [PubMed]
- Cantini L, Hassan R, Sterman DH, et al. Emerging Treatments for Malignant Pleural Mesothelioma: Where Are We Heading? Front Oncol 2020;10:343. [Crossref] [PubMed]
- Wang Z, Wang XY, Li J, et al. Prognostic and Clinicopathological Significance of BAP1 Protein Expression in Different Types of Cancer-A Meta-Analysis. Genet Test Mol Biomarkers 2018;22:115-26. [Crossref] [PubMed]
- Guazzelli A, Meysami P, Bakker E, et al. BAP1 Status Determines the Sensitivity of Malignant Mesothelioma Cells to Gemcitabine Treatment. Int J Mol Sci 2019;20:429. [Crossref] [PubMed]
- Okonska A, Bühler S, Rao V, et al. Functional Genomic Screen in Mesothelioma Reveals that Loss of Function of BRCA1-Associated Protein 1 Induces Chemoresistance to Ribonucleotide Reductase Inhibition. Mol Cancer Ther 2020;19:552-63. [Crossref] [PubMed]
- Hassan R, Morrow B, Thomas A, et al. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc Natl Acad Sci U S A 2019;116:9008-13. [Crossref] [PubMed]
- Baumann F, Flores E, Napolitano A, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 2015;36:76-81. [Crossref] [PubMed]
- Pastorino S, Yoshikawa Y, Pass HI, et al. A Subset of Mesotheliomas With Improved Survival Occurring in Carriers of BAP1 and Other Germline Mutations. J Clin Oncol 2018;36:JCO2018790352 [Crossref] [PubMed]
- Panou V, Gadiraju M, Wolin A, et al. Frequency of Germline Mutations in Cancer Susceptibility Genes in Malignant Mesothelioma. J Clin Oncol 2018;36:2863-71. [Crossref] [PubMed]
- Farzin M, Toon CW, Clarkson A, et al. Loss of expression of BAP1 predicts longer survival in mesothelioma. Pathology 2015;47:302-7. [Crossref] [PubMed]
- Cantini L, Pecci F, Murrone A, et al. Questioning the prognostic role of BAP-1 immunohistochemistry in malignant pleural mesothelioma: A single center experience with systematic review and meta-analysis. Lung Cancer 2020;146:318-26. [Crossref] [PubMed]
- Forest F, Patoir A, Dal Col P, et al. Nuclear grading, BAP1, mesothelin and PD-L1 expression in malignant pleural mesothelioma: prognostic implications. Pathology 2018;50:635-41. [Crossref] [PubMed]
- De Rienzo A, Archer MA, Yeap BY, et al. Gender-Specific Molecular and Clinical Features Underlie Malignant Pleural Mesothelioma. Cancer Res 2016;76:319-28. [Crossref] [PubMed]
- Yoshikawa Y, Sato A, Tsujimura T, et al. Frequent inactivation of the BAP1 gene in epithelioid-type malignant mesothelioma. Cancer Sci 2012;103:868-74. [Crossref] [PubMed]
- Laurila EM, Kallioniemi A. The diverse role of miR-31 in regulating cancer associated phenotypes. Genes Chromosomes Cancer 2013;52:1103-13. [Crossref] [PubMed]
- Stepicheva NA, Song JL. Function and regulation of microRNA-31 in development and disease. Mol Reprod Dev 2016;83:654-74. [Crossref] [PubMed]
- Yu T, Ma P, Wu D, et al. Functions and mechanisms of microRNA-31 in human cancers. Biomed Pharmacother 2018;108:1162-9. [Crossref] [PubMed]
- Ivanov SV, Goparaju CM, Lopez P, et al. Pro-tumorigenic effects of miR-31 loss in mesothelioma. J Biol Chem 2010;285:22809-17. [Crossref] [PubMed]
- Moody HL, Lind MJ, Maher SG. MicroRNA-31 Regulates Chemosensitivity in Malignant Pleural Mesothelioma. Mol Ther Nucleic Acids 2017;8:317-29. [Crossref] [PubMed]
- Matsumoto S, Nabeshima K, Hamasaki M, et al. Upregulation of microRNA-31 associates with a poor prognosis of malignant pleural mesothelioma with sarcomatoid component. Med Oncol 2014;31:303. [Crossref] [PubMed]
- Ma M, Yu N, Wu B. High systemic immune-inflammation index represents an unfavorable prognosis of malignant pleural mesothelioma. Cancer Manag Res 2019;11:3973-9. [Crossref] [PubMed]
- Nicholson AG, Sauter JL, Nowak AK, et al. EURACAN/IASLC Proposals for Updating the Histologic Classification of Pleural Mesothelioma: Towards a More Multidisciplinary Approach. J Thorac Oncol 2020;15:29-49. [Crossref] [PubMed]


