
Pyruvate dehydrogenase E1α represents a reliable prognostic predictor for patients with non-small cell lung cancer resected via curative operation
Introduction
Lung cancer has a high morbidity and mortality rate worldwide, and is associated with the poor prognosis despite recent advances in therapy (1). Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers. Several clinicopathological prognostic factors, such as pathological stage, mutations in genes, and smoking history, have been reported to be associated with the outcome after surgery in patients with NSCLC (2,3); however, no reliable and independent prognostic predictor for NSCLC after curative surgery has been identified at present.
Glucose metabolism in cancer cells is increased considerably with increasing proliferation (4,5). In particular, anaerobic glycolysis is dominant even under aerobic conditions in the tumor microenvironment, which is known as the Warburg effect (6), suggesting that the enzymes involved in glucose metabolism in cancer cells may represent an important indicator of cancer malignancy (7,8). Among the glucose metabolism enzymes, the pyruvate dehydrogenase (PDH) complex catalyzes the conversion of pyruvate to acetyl-CoA and promotes aerobic glucose metabolism; PDH is phosphorylated by PDH kinase (PDK). The PDH-E1 enzyme, which is inhibited and regulated by the PDH complex, consists of a hetero-tetramer containing two α subunits and two β subunits. The E1-α subunit (PDH-E1α) contains the E1 active site and plays a key role in the function of the PDH complex (9). PDK activity has been reported in several types of carcinomas, such as colorectal cancer, bladder cancer, pancreatic cancer, and NSCLC (10-13). However, few studies have examined the relationship between PDH-E1α expression and clinicopathological factors associated with NSCLC. In this study, we aimed to determine whether PDH-E1α may serve as a prognostic predictor for NSCLC after curative surgery.
We present the following article in accordance with the REMARK reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-1463).
Methods
Patient selection and ethical statement
We retrospectively investigated the clinical course of 665 patients with NSCLC who underwent surgery at Osaka City University, Osaka, Japan, between January 2010 and December 2016. Patients with R1 or R2 surgery, with preoperative chemotherapy or radiation therapy, and patients without curative resection, such as wedge resection, segmentectomy, and lobectomy without mediastinal lymph node dissection, were excluded. A total of 445 patients with histologically confirmed primary NSCLC with pathological stage 0 to stage IIIA who underwent R0 surgery (more than lobectomy and mediastinal lymph node dissection) were enrolled in this study. Pathological findings were determined according to 8th edition of the Union for International Cancer Control TNM classification. After final staging, the administration and regimen of adjuvant chemotherapy was appropriately determined by the cancer board consisting of a thoracic surgeon, a radiologist, and an oncologist. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Osaka City University Ethics Committee (Reference number 2019-006). Informed consent was obtained from all patients. All methods with respect to humans performed in accordance with the relevant guidelines and regulations.
Immunostaining of the PDH-E1α
In brief, paraffin-embedded sections from 445 patients were deparaffinized in xylene and hydrated in decreasing concentrations of ethyl alcohol. The sections were incubated with 3% hydrogen peroxide to block endogenous peroxidase activity. The sections were then heated for 10 min at 105 °C by autoclave in Target Retrieval Solution (DAKO, Carpinteria, CA, USA). Nonspecific binding was blocked via incubation with 10% normal rabbit serum for 10 min. The specimens were incubated with anti PDH-E1α antibody (sc-377092; 1:100; Santa Cruz Biotechnology, Dallas, TX, USA; RRID:AB_2716767) (14) at 4 °C overnight. These sections were incubated with a mouse linker for 10 min, and peroxidase-labeled polymer (Histofine SAB-PO(M) kit, Nichirei Biosciences Inc, Tokyo, Japan) for 5 min, followed by counterstaining with Mayer’s hematoxylin.
Immunohistochemical determination
The immunoreactivity of PDH-E1α was evaluated according to the intensity of membranous staining at the deepest level of the tumor and the proportion of immunoreactive cells. Immunostaining intensity score was rated 0–3 as follows: 0, negative; 1+, weakly positive; 2+, positive; 3+, strongly positive (Figure 1). The immunostaining proportion score was measured as an estimate of the proportion of positive cells: 0, no immunoreactive cells; 1+, <30% immunoreactive cells; 2+, 40–70% immunoreactive cells; 3+, >80% immunoreactive cells. The total score was calculated as the summation of the immunostaining intensity score and proportion score ranging from 0 to 6, and PDH-E1α expression was considered positive when the summation score was ≥4.
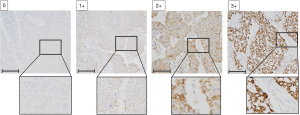
Statistical analysis
The χ2 test was performed to determine the significance of differences between covariates. Survival durations were calculated using the Kaplan-Meier method and analyzed using the log-rank test to compare the cumulative survival durations in the patient groups. In addition, the Cox proportional hazards model was used to compute the multivariate hazard ratios for the study parameters. In all tests, a P value <0.05 was considered significant. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface of R, and more precisely, a modified version of the R commander (The R Foundation for Statistical Computing, Vienna, Austria) (15).
Results
Relationship between PDH-E1α expression and clinicopathological features
The clinicopathological features of all 445 patients based on PDH-E1α expression are summarized in Table 1. In total, 248 patients (56%) of the 445 patients with NSCLC patients were PDH-E1α-positive and 197 patients were PDH-E1α-negative. PDH-E1α positivity was significantly correlated with the presence of adenocarcinoma (P<0.001) in comparison to the PDH-E1α-negative group. No other clinicopathological features were correlated with PDH-E1α expression, including induction of adjuvant chemotherapy and postoperative complications. There was no significant correlation between PDH-E1α expression and clinicopathological features in patients with adenocarcinoma or with squamous cell carcinoma, respectively.
Table 1
| Variables | Subgroup | PDH-E1α expression | ||
|---|---|---|---|---|
| Positive (n=248), n (%) | Negative (n=197), n (%) | P value | ||
| Age | <65 | 83 (33.5) | 53 (26.9) | 0.148 |
| ≥65 | 165 (66.5) | 144 (73.1) | ||
| Sex | Female | 95 (38.3) | 60 (30.5) | 0.089 |
| Male | 153 (61.7) | 137 (69.5) | ||
| Smoking | Yes | 178 (71.8) | 135 (68.5) | 0.466 |
| No | 70 (28.2) | 62 (31.5) | ||
| Histology | Adenocarcinoma | 185 (74.6) | 114 (57.9) | <0.001 |
| Squamous cell carcinoma | 50 (20.2) | 70 (35.5) | ||
| Others | 13 (5.2) | 13 (6.6) | ||
| Lymphatic invasion | Negative | 168 (67.7) | 137 (69.5) | 0.758 |
| Positive | 80 (32.3) | 60 (30.5) | ||
| Venous invasion | Negative | 211 (85.1) | 155 (78.7) | 0.082 |
| Positive | 37 (14.9) | 42 (21.3) | ||
| Pleural invasion | Negative | 185 (74.6) | 146 (74.1) | 0.913 |
| Positive | 63 (25.4) | 51 (25.9) | ||
| Depth of invasion | T1 or T2 | 218 (87.9) | 166 (84.3) | 0.271 |
| T3 or T4 | 30 (12.1) | 31 (15.7) | ||
| Lymph node metastasis | N0 | 199 (80.2) | 147 (74.6) | 0.169 |
| N1 or N2 | 49 (19.8) | 50 (25.4) | ||
| pStage | 0–II | 208 (83.9) | 157 (79.7) | 0.266 |
| III | 40 (16.1) | 40 (20.3) | ||
PDH-E1α, pyruvate dehydrogenase E1α; NSCLC, non-small cell lung carcinoma.
Correlation between PDH-E1α expression and survival of patients with NSCLC
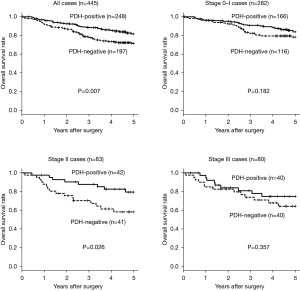
The 5-year overall survival rate according to PDH-E1α expression in all 445 patients is presented in Figure 2. Patients with NSCLC showing PDH-E1α-negative expression had significantly poorer survival rates (P=0.007) than those of patients showing PDH-E1α-positive expression. According to the pathological stages, the 5-year overall survival rate of patients with NSCLC showing PDH-E1α-negative expression was a significantly poorer (P=0.026) than those showing PDH-E1α-positive expression in stage II. In contrast, no significant difference was found between PDH-E1α expression in stages 0–I and III.
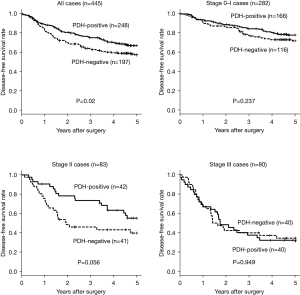
The 5-year disease-free survival rate, according to PDH-E1α expression in all 445 patients, is presented in Figure 3. Patients with NSCLC showing PDH-E1α-negative expression had significantly poorer survival rates (P=0.02) than patients showing PDH-E1α-positive expression. According to the pathological stages, no significant difference was found between PDH-E1α expression in all stages.
There was no significant correlation between PDH-E1α expression and overall and disease-free survival rates in patients with adenocarcinoma or squamous cell carcinoma, respectively.
Univariate and multivariate analyses of survival
The results of the univariate and multivariate analyses of the overall survival are presented in Table 2. Univariate analysis showed that poor overall survival was significantly correlated with PDH-E1α negativity (P=0.007), male sex (P<0.001), smoking (P=0.049), histology of adenocarcinoma (P=0.001), lymphatic invasion (P=0.006), pleural invasion (P=0.002), pathological T3/4 (P=0.02), and lymph node metastasis (P=0.014). Multivariate analysis revealed that PDH-E1α negativity (P=0.037) and male sex (P<0.001) were significantly correlated with poor overall survival.
Table 2
| Variables | Subgroup | Number | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||||
| Sex | Female | 155 | 4.99 | 2.66–9.37 | <0.001 | 4.03 | 2.11–7.7 | <0.001 | |
| Male | 290 | ||||||||
| Age | <65 | 136 | 1.37 | 0.87–2.17 | 0.175 | ||||
| ≥65 | 309 | ||||||||
| Smoking | Yes | 313 | 1.52 | 1.00–2.29 | 0.049 | 1.38 | 0.91–2.09 | 0.132 | |
| No | 132 | ||||||||
| Histology | Adenocarcinoma | 299 | 1.94 | 1.29–2.91 | 0.001 | 1.21 | 0.79–1.86 | 0.375 | |
| Others | 146 | ||||||||
| Lymphatic invasion | Negative | 305 | 1.78 | 1.18–2.67 | 0.006 | 1.22 | 0.77–1.94 | 0.394 | |
| Positive | 140 | ||||||||
| Venous invasion | Negative | 366 | 1.38 | 0.84–2.29 | 0.206 | ||||
| Positive | 79 | ||||||||
| Pleural invasion | Negative | 331 | 1.94 | 1.27–2.94 | 0.002 | 1.5 | 0.94–2.38 | 0.086 | |
| Positive | 114 | ||||||||
| Pathological T | 1 or 2 | 384 | 1.82 | 1.1–3.01 | 0.02 | 1.1 | 0.64–1.88 | 0.729 | |
| 3 or 4 | 61 | ||||||||
| Pathological N | 0 | 346 | 1.74 | 1.12–2.7 | 0.014 | 1.22 | 0.76–1.96 | 0.419 | |
| 1 or 2 | 99 | ||||||||
| Adjuvant chemotherapy | Yes | 119 | 1.25 | 0.78–2.02 | 0.355 | ||||
| No | 326 | ||||||||
| Postoperative complications | Yes | 63 | 1.39 | 0.72–2.68 | 0.323 | ||||
| No | 382 | ||||||||
| PDH-E1α expression | Negative | 197 | 1.74 | 1.16–2.61 | 0.007 | 1.55 | 1.03–2.33 | 0.037 | |
| Positive | 248 | ||||||||
NSCLC, non-small cell lung carcinoma; CI, confidence interval; HR, hazard ratio; PDH–E1α, pyruvate dehydrogenase E1α.
Table 3 shows the univariate and multivariate analyses results of the disease-free survival. Univariate analysis showed that a poor disease-free survival was significantly correlated with PDH-E1α negativity (P=0.021), male sex (P<0.001), histology of adenocarcinoma (P=0.029), venous invasion (P<0.001), lymphatic invasion (P<0.001), pleural invasion (P<0.001), pathological T3/4 (P<0.001), lymph node metastasis (P<0.001), and adjuvant chemotherapy (P=0.005). Multivariate analysis revealed that male sex (P=0.002), pleural invasion (P=0.002), and lymph node metastasis (P<0.001) were significantly correlated with a poor disease-free survival, while PDH-E1α negativity was not (P=0.068).
Table 3
| Variables | Subgroup | Number | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||||
| Sex | Female | 155 | 2.42 | 1.66–3.54 | <0.001 | 1.86 | 1.25–2.76 | 0.002 | |
| Male | 290 | ||||||||
| Age | <65 | 136 | 1.29 | 0.92–1.83 | 0.144 | ||||
| ≥65 | 309 | ||||||||
| Smoking | Yes | 313 | 1.29 | 0.93–1.78 | 0.121 | ||||
| No | 132 | ||||||||
| Histology | Adenocarcinoma | 299 | 1.42 | 1.04–1.95 | 0.029 | 0.84 | 0.59–1.19 | 0.318 | |
| Others | 146 | ||||||||
| Lymphatic invasion | Negative | 305 | 2.36 | 1.74–3.22 | <0.001 | 1.29 | 0.9–1.85 | 0.168 | |
| Positive | 140 | ||||||||
| Venous invasion | Negative | 366 | 2.28 | 1.61–3.22 | <0.001 | 1.21 | 0.83–1.77 | 0.332 | |
| Positive | 79 | ||||||||
| Pleural invasion | Negative | 331 | 2.53 | 1.84–3.46 | <0.001 | 1.77 | 1.23–2.55 | 0.002 | |
| Positive | 114 | ||||||||
| Pathological T | 1 or 2 | 384 | 2.31 | 1.6–3.34 | <0.001 | 1.45 | 0.95–2.2 | 0.087 | |
| 3 or 4 | 61 | ||||||||
| Pathological N | 0 | 346 | 3.1 | 2.26–4.27 | <0.001 | 2.42 | 1.66–3.53 | <0.001 | |
| 1 or 2 | 99 | ||||||||
| Adjuvant chemotherapy | Yes | 119 | 0.63 | 0.45–0.86 | 0.005 | 1.21 | 0.84–1.75 | 0.304 | |
| No | 326 | ||||||||
| Postoperative complications | Yes | 63 | 1.22 | 0.76–1.95 | 0.409 | ||||
| No | 382 | ||||||||
| PDH–E1α expression | Negative | 197 | 1.44 | 1.06–1.95 | 0.021 | 1.34 | 0.98–1.83 | 0.068 | |
| Positive | 248 | ||||||||
NSCLC, non-small cell lung carcinoma; CI, confidence interval; HR, hazard ratio; PDH–E1α, pyruvate dehydrogenase E1α.
Discussion
Lung cancer is associated with a high morbidity and mortality rate worldwide; however, no reliable and independent prognostic predictor for NSCLC after curative surgery is available. PDH activity has been found to be associated with various cancers. However, few studies have evaluated the relationship between PDH-E1α expression and clinicopathological factors associated with NSCLC. In this study, PDH-E1α expression in NSCLC was found to be significantly correlated with the presence of adenocarcinoma. It has been reported that histological adenocarcinoma type is associated with a higher oxygen-containing environment than that of squamous cell-type carcinoma in NSCLC (16). Normal cells obtain energy from glucose via mitochondrial oxidative phosphorylation under aerobic conditions (17). These findings suggest that the expression level of PDH-E1α might have been higher in adenocarcinoma than in squamous cell carcinoma due to the relatively higher oxygen levels in the adenocarcinoma environment.
A cell produces 34 molecules of adenosine triphosphate (ATP) from a single molecule of glucose through the glycolysis to TCA cycle and the electron transfer system under normoxic conditions, called oxidative phosphorylation. On the other hand, a single molecule of glucose provides two molecules of ATP in a hypoxic environment, called anaerobic glycolysis. These two different metabolic pathways diverge at pyruvate. PDH-E1α catalyzes the conversion of pyruvate to acetyl-CoA, which enters into the TCA cycle, and PDH-E1α negativity induces anaerobic glycolysis. As anaerobic glycolysis is upregulated in cancer cells, extracellular acidosis is caused by the accumulation of lactate (18). Normal cells show a low tolerance to acidosis; however, cancer cells can readily adapt to acidosis via mutations such as in p53, and thereby continue to proliferate (19). Furthermore, when metastatic cancer cells proliferate by occluding the intravascular space, the tolerance to hypoxic environments and acidosis is advantageous to cancer cells (20). These findings suggest that anaerobic glycolysis may be upregulated and PDH-E1α expression may be downregulated in cancer cells with malignancy potential.
Patients with NSCLC showing PDH-E1α expression were found to have significantly higher overall survival rates than those showing PDH-E1α-negative expression in pathological stage II. PDH-E1α expression may serve as an independent prognostic factor in patients with NSCLC treated with R0 resection, and was not significantly correlated with postoperative complications or administration of adjuvant chemotherapy. Low PDH-E1α levels in cancer cells are closely related to the malignancy potential of cancer cells, such as distant metastasis (20), suggesting that PDH-E1α may represent a reliable prognostic predictor for patients with NSCLC after curative operation, especially at stage II.
However, PDH-E1α-negative expression had a significantly poorer survival rate in pathological stage II only, not in stages 0–I and III. This is probably because the expression of PDH-E1α indicates the potential of cancer malignancy. In patients with pathological stage 0–I NSCLC, the good effect of surgery for prognosis may be larger than the malignant potential of PDH negativity. On the other hand, the prognosis of patients with pathological stage IIIA NSCLC may have been significantly affected by cancer progression rather than by the malignant potential of PDH-E1α negativity.
To the best of our knowledge, this is the first report to shows that PDH-E1α represents a reliable prognostic predictor for patients with NSCLC resected via curative operation. These findings suggest that PDH-E1α plays an important role in NSCLC development. PDH-E1α or PDK may serve as potential target in the treatment of NSCLC (21-23). Further investigation on the regulation of PDH-E1α expression may assist in identifying a promising therapeutic target for effective treating stage II NSCLC.
This study has some limitations. First, this is a retrospective study. It was not possible to analyze all factors in all patients, and some patients dropped out of the trial and could not be followed up upon. Second, we evaluated PDH-E1α expression only, but did not include all PDH subunits such as E1β, E2, or E3. In addition, PDH-E1α expression was evaluated only by immunohistochemistry. The accumulation of gene or mRNA data would be ideal for supporting the results of this study.
In conclusion, PDH-E1α represents a reliable prognostic predictor for patients with NSCLC undergoing curative resection. PDK may serve as a promising target for patients with NSCLC.
Acknowledgments
Funding: This study was supported by KAKENHI (Grant-in-Aid for Scientific Research) [21H03008].
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-1463
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-1463
Peer Review File: Available at https://dx.doi.org/10.21037/jtd-21-1463
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-1463). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Osaka City University Ethics Committee (Reference number 2019-006) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Abdel-Wahab AF, Mahmoud W, Al-Harizy RM. Targeting glucose metabolism to suppress cancer progression: prospective of anti-glycolytic cancer therapy. Pharmacol Res 2019;150:104511 [Crossref] [PubMed]
- Lebelo MT, Joubert AM, Visagie MH. Warburg effect and its role in tumourigenesis. Arch Pharm Res 2019;42:833-47. [Crossref] [PubMed]
- . WARBURG O. On the origin of cancer cells. Science 1956;123:309-14. [Crossref] [PubMed]
- Thews O, Riemann A. Tumor pH and metastasis: a malignant process beyond hypoxia. Cancer Metastasis Rev 2019;38:113-29. [Crossref] [PubMed]
- Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab 2016;23:27-47. [Crossref] [PubMed]
- Ozden O, Park SH, Wagner BA, et al. SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells. Free Radic Biol Med 2014;76:163-72. [Crossref] [PubMed]
- Yang Z, Zhang SL, Hu X, et al. Inhibition of pyruvate dehydrogenase kinase 1 enhances the anti-cancer effect of EGFR tyrosine kinase inhibitors in non-small cell lung cancer. Eur J Pharmacol 2018;838:41-52. [Crossref] [PubMed]
- Jin L, Kim EY, Chung TW, et al. Hemistepsin A suppresses colorectal cancer growth through inhibiting pyruvate dehydrogenase kinase activity. Sci Rep 2020;10:21940. [Crossref] [PubMed]
- Woolbright BL, Choudhary D, Mikhalyuk A, et al. The Role of Pyruvate Dehydrogenase Kinase-4 (PDK4) in Bladder Cancer and Chemoresistance. Mol Cancer Ther 2018;17:2004-12. [Crossref] [PubMed]
- Tambe Y, Terado T, Kim CJ, et al. Antitumor activity of potent pyruvate dehydrogenase kinase 4 inhibitors from plants in pancreatic cancer. Mol Carcinog 2019;58:1726-37. [Crossref] [PubMed]
- Yang Z, Wang Y, Zhang L, et al. Phosphorylated form of pyruvate dehydrogenase α1 mediates tumor necrosis factor α-induced glioma cell migration. Oncol Lett 2021;21:176. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Schuurbiers OC, Meijer TW, Kaanders JH, et al. Glucose metabolism in NSCLC is histology-specific and diverges the prognostic potential of 18FDG-PET for adenocarcinoma and squamous cell carcinoma. J Thorac Oncol 2014;9:1485-93. [Crossref] [PubMed]
- Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci 2016;73:377-92. [Crossref] [PubMed]
- de la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, et al. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front Oncol 2019;9:1143. [Crossref] [PubMed]
- Lamonte G, Tang X, Chen JL, et al. Acidosis induces reprogramming of cellular metabolism to mitigate oxidative stress. Cancer Metab 2013;1:23. [Crossref] [PubMed]
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004;4:891-9. [Crossref] [PubMed]
- Anwar S, Mohammad T, Shamsi A, et al. Discovery of Hordenine as a Potential Inhibitor of Pyruvate Dehydrogenase Kinase 3: Implication in Lung Cancer Therapy. Biomedicines 2020;8:119. [Crossref] [PubMed]
- Lee EJ, Chung TW, Lee JH, et al. Water-extracted branch of Cinnamomum cassia promotes lung cancer cell apoptosis by inhibiting pyruvate dehydrogenase kinase activity. J Pharmacol Sci 2018;138:146-54. [Crossref] [PubMed]
- Guo F, Zhao S, Li X. Discovery of novel pyruvate dehydrogenase kinases inhibitors by screening of an in-house small molecule library for anti-lung cancer therapeutics. Bioorg Med Chem Lett 2019;29:291-6. [Crossref] [PubMed]


