Partial esophagogastrostomy with esophagogastric anastomosis below the aortic arch in cardiac carcinoma: characteristics and treatment of postoperative anastomotic leakage
Introduction
The most common surgical approach for cardiac cancer is partial esophagogastrostomy through the left thorax, and esophagogastric anastomosis below aortic arch (>90% of cases). Anastomotic leakage is the major complication after surgery, and the major cause of perioperative death. The reported incidence of anastomotic leak varied, with the highest up to 50% (1) and mortality being 30% to 60% (2-4).
With advances in anastomotic technology and perioperative management, the morbidity and mortality of anastomotic leakage has decreased significantly in recent years. However, due to the variations in the clinical manifestations and severity of intrathoracic anastomotic leak, complex and diverse subsequent pathophysiological changes, and dependent optimal approach on the doctor-patient matrix, the clinical diagnosis and treatment decision-making is still a challenge for clinicians.
In this study, we retrospectively reviewed the clinical data of 25 cardiac carcinoma patients with postoperative anastomotic leakage after surgery in the Department of Thoracic Surgery, Chinese Academy of Medical Sciences Cancer Hospital between January 2009 and December 2013. We analyzed the clinical characteristics, diagnostic methods, and individualized treatment based on their clinical manifestations.
Materials and methods
General information
From January 2009 to December 2013, 1,196 patients with cardiac carcinoma underwent partial esophagogastrostomy via the left thorax with esophagogastric anastomosis below the aortic arch in the Department of Thoracic Surgery, Chinese Academy of Medical Sciences Cancer Hospital. Among them, 25 (2.09%, 25/1,196) had significant post-operative anastomotic leakage.
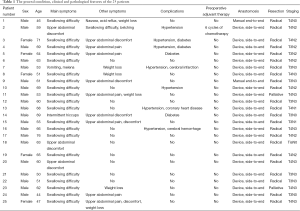
Among the 25 patients, there are 20 men and 5 women with median age of 61 (range, 44-76). Preoperative manifestations included dysphagia in 21 cases, abdominal discomfort or pain in 12 cases, intermittent hiccups in 1 case, hematemesis and black stools in 1 case and weight loss in 6 cases. Complication included hypertension in 7 cases and diabetes mellitus in 4. One patient received 6 cycles of preoperative chemotherapy (Table 1).
Full table
Preoperative examination, surgery and pathology
All patients received preoperative routine examination and were diagnosed as cardiac carcinoma by endoscopic biopsy. The extent and scope of the disease involvement was determined tumor resectability and gastroesophageal reconstruction feasibility. Eligible patients underwent partial esophagogastrostomy via the left thorax with esophagogastric anastomosis below the aortic arch. Except for two patients who received manual end-to-end anastomosis, the remaining 23 patients underwent gastroesophageal end-to-side anastomosis with instrument. Palliative resection was performed in 2 cases, and radical resection in 23 cases. Pathological diagnosis revealed adenocarcinoma in all cases: stage 0 in 1 case (TisN0M0), IIB in 2 cases (T3N0M0), IIIA in 2 cases (T4N0M0, T3N1M0 each), IIIB in 2 cases (T3N2M0), and IIIC in 18 cases (5, 1, 6 and 6 cases at T3N3M0, T4N1M0, T4N2M0, T4N3M0, respectively).
Results
Manifestations of anastomotic leakage
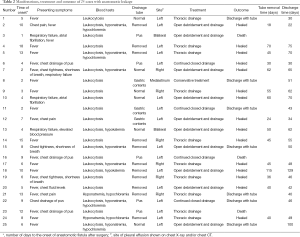
The median time to the initial symptoms of anastomotic leak was 6 days (1-18 days). There were 21 cases of high fever, 9 cases of chest pain, dyspnea and chest tightness (including 4 cases of respiratory failure), and 4 cases of gastric juice and/or purulent fluid from the chest tubes. Elevated leukocyte levels were present as the onset symptom in 22 cases, while hyponatremia and/or hypochloremia were present in 9 cases (Table 2). At the time of onset, 11 cases had their chest tubes removed, 4 cases were diagnosed due to gastric juice and/or purulent fluid from the chest closed drainage tubes, and 6 had no gastric juice and/or purulent fluid despite retention of the drainage. Bedside chest X-ray and/or chest CT showed left pleural effusions in 16 cases (including 7 cases of significant gas-liquid surface), right pleural effusions in 6 cases (including 3 cases of significant gas-liquid surface), bilateral pleural effusions in 2 cases and mediastinal encapsulated effusion in 1 case (Table 2). Among the 7 cases undergoing anastomotic imaging, 6 were found to have contrast extravasation at the anastomosis, and 12 patients who underwent endoscopy were found to have local anastomotic fistula.
Full table
Treatment of anastomotic leakage
Once anastomotic leakage is confirmed, patients were subjected to strict water fasting and gastrointestinal decompression with placement of the small intestine nasogastric feeding tube and/or intravenous nutrition supplement and, where appropriate, intravenous antibiotics depending on the infection situation.
Patency was remained for closed thoracic drainage in the 4 cases with gastric juice and/or purulent fluid from the chest tubes. Among the 12 patients with left chest infections confirmed by transthoracic puncture, 5 received ultrasound-guided left thoracic closed drainage, and 7 underwent thoracotomy of the original incisions for debridement and drainage. All of the 6 patients with right chest infections confirmed by transthoracic puncture received ultrasound-guided right thoracic closed drainage, and one of them underwent thoracotomy of the original incisions for debridement and drainage via the left thorax. All of the 2 patients with bilateral chest infections confirmed by transthoracic puncture received ultrasound-guided right thoracic closed drainage and thoracotomy of the original incisions for debridement and drainage via the left thorax. Conservative treatment was prescribed for one case with mediastinal infection (Table 2).
Clinical outcomes
After treatment, three patients died, among which two who had left pleural infection and received left pleural drainage died of gastrointestinal bleeding 11 and 52 days after the procedure, respectively. The other patient with bilateral pleural infection died on 175th day due to repeated infection with multiple organ failure despite right thoracic drainage and left open drainage. Of the remaining 22 patients, 15 patients showed anastomotic healing after treatment, the tube removal time was 18-115 days after surgery with a median time of 45 days; the time to discharge was 30-129 days with a median time of 49 days after surgery. The seven patients who did not have their leakage healed had formed chronic infectious fistulas, and were discharged with nasogastric feeding tubes and chest tubes 30-100 days after surgery, with a median time to discharge of 46 days after surgery without any symptoms of systemic infection (Table 2).
Discussion
Healing of the esophagogastric anastomosis after surgery for cardiac carcinoma patients is affected by many systemic and local factors. Systemic factors include malnutrition, anemia, hypotension, hypoxia, neoadjuvant therapy, diabetes, atherosclerosis, and so on, which should be treated carefully during the perioperative period. Local factors include: (I) compared with other digestive organs, the esophagus has no serosa and is mainly composed of longitudinal muscle layers, making it lack of suture strength and healing ability; (II) the esophageal blood supply is staged as the transportation branches extend to longer distances, making it vulnerable to ischemic necrosis in surrounding tissue at the anastomosis; (III) negative intrathoracic pressure may also lead to leakage of gastric juice from the manual or anastomotic suture into the chest; (IV) the blood supply of the gastric remnant derives from the right gastroepiploic artery, which may cause supply deficiencies at the anastomosis after the stomach is lifted to the chest. Prospective studies have shown that (5,6) there is no difference in the incidence of anastomotic leakage between single-layer continuous suture and single-layer interrupted suture, or between manual and stapler single-layer continuous suture. We believe that regardless of manual or mechanical anastomosis, gentle actions are required to avoid damage and tear, thereby reducing the incidence of anastomotic fistula. Studies have shown that (7-13) preventive placement of anastomotic drainage tubes may be conducive to early detection and control of anastomotic leakage. Using pedicle-based omentum for anastomotic reinforcement may reduce the incidence of anastomotic fistula and extent of mediastinal contamination.
The manifestations and severity of intrathoracic anastomotic leak are very heterogeneous. Those with gastric juice spills from the chest tube and secondary mediastinal and pleural cavity infections may be called the “dominant anastomotic leakage,” and those with insignificant clinical manifestations what can only be shown in imaging or endoscopy may be called “hidden anastomotic leakage” (14). Lerut et al. suggested the Lerut grading based on the severity of symptoms of anastomotic leakage (1). In the present study, all of the 25 patients had the “dominant anastomotic leakage” with an incidence of 2.09%, while the so-called “hidden anastomotic leakage” could not be accurately studied due to subclinical manifestations and minor impact on the clinical diagnosis and treatment decisions.
The esophagogastric anastomosis below the aortic arch is located at the posterior mediastinum, adjacent to the pericardium, the descending aorta, the prevertebral fascia, and bilateral pleural cavities. In the case of anastomotic leakage, the leakage of stomach contents and secondary suppurative inflammation may affect unilateral or bilateral pleural cavities and may also spread to the mediastinum or be confined in the mediastinal esophageal bed. Gastroesophageal anastomotic leakage can cause pleural cavity contamination, infection, and obstruction, affecting the pleural cavity negative pressure, causing severe respiratory dysfunction and hemodynamic instability, which are different from other systemic inflammatory symptoms caused by gastrointestinal fistulas. The clinical manifestations are serious and often fatal.
According to the long-term experience of the Department of Thoracic Surgery, Chinese Academy of Medical Sciences Cancer Hospital, we propose to classify the postoperative esophageal anastomotic leakage into the following five types to facilitate the clinical diagnosis and treatment decisions.
(I) Occult type: despite clinical suspicion of a small fistula, the clinical manifestations are not serious or typical. Patients are generally in good condition; and infection symptoms are not severe. In the case of increased body temperature after liquid diet, strict fasting, gastrointestinal decompression and appropriate antibiotic use can help to reduce the body temperature to normal. The increased body temperature is not manifested as persistent fever, and can be relieved with intermittent use of antipyretics, or physical cooling, or can be self-limiting. Leukocytes and/or neutrophils can be mildly to moderately increased, while electrolytes and biochemistry are normal. Imaging (chest X-ray and/or chest CT) findings include normal bilateral pleural cavities without obvious liquid surface. Even if there is pleural effusion, there is no obvious evidence of infection by thoracentesis. Through strict fasting, gastrointestinal decompression, nasogastric enteral nutrition and/or intravenous nutrition, appropriate empiric use of antibiotics, and symptomatic treatment, such anastomotic leakage is likely to heal by itself. The presence and healing of this anastomotic leakage can be confirmed by endoscopy and/or imaging, but it has not much to do with further treatment strategies and outcomes. The endoscopy and/or imaging can also be waived and water and liquid diet given gradually one week after the body temperature recovers to normal to confirm healing;
(II) Left thoracic type: the anastomotic leakage mainly penetrates to the left chest, with left chest infection symptoms and/or gastric juice and/or purulent fluid appearing in the thoracic drainage tubes. Based on the severity of the infection, chest pain and systemic symptoms (such as fever, cold sweats, increased heart rate and pulse rate, decreased blood pressure, respiratory failure, etc.) can be present, as well as increased leukocytes and/or neutrophils, electrolyte biochemical abnormalities such as decreased sodium, serum chloride ion and hypoalbuminemia. Imaging (chest X-ray and/or chest CT) may show left pleural effusion, especially liquid surface, which has diagnostic value, and presence of gastric juice and/or purulent fluid; blue liquid from thoracentesis or pleural drainage after oral methylene blue is diagnostic. Endoscopy and/or contrast imaging can confirm the existence of anastomotic leakage on the left. In the present cohort, patients number 1, 2, 4, 5, 6, 10, 11, 12, 15, 16, 18, 20, 22, 23, 24 and 25 were the left thoracic type. For the left thoracic type anastomotic leakage, depending on the severity, the treatment methods include: thoracentesis or pleural puncture catheter drainage (which can be supplemented by pleural lavage), thoracic closed drainage, and thoracotomy for debridement and drainage. Other treatment measures include strict fasting, gastrointestinal decompression, nasogastric enteral nutrition and/or intravenous nutrition, appropriate empiric use of antibiotics, and symptomatic treatment such as proton pump inhibitors and somatostatin to reduce gastric acid secretion. After the patient’s body temperature returns to normal and blood tests are normal without drainage discharge after oral methylene blue, and chest radiographic examination shows no significant fluid levels, and endoscopy and/or imaging confirms fistula healing, we can consider gradually giving water, liquid diet, and after further verifying the healing, the chest tube can be removed step by step;
(III) Right thoracic type: the anastomotic leakage mainly penetrates to the right chest, with right pleural infection on clinical symptoms (similar to the left chest type). Imaging (chest X-ray and/or chest CT) may show right pleural effusion, especially liquid surface, which has diagnostic value, and presence of gastric juice and/or purulent fluid; blue liquid from thoracentesis or pleural drainage after oral methylene blue is diagnostic. Endoscopy and/or contrast imaging can confirm the existence of anastomotic leakage on the right. In the present cohort, patients number 7, 9, 14, 17, 19 and 21 were the right thoracic type. Depending on the severity, the treatment methods include: thoracentesis or pleural puncture catheter drainage (which can be supplemented by pleural lavage), and thoracic closed drainage. Other treatment measures include strict fasting, gastrointestinal decompression, nasogastric enteral nutrition and/or intravenous nutrition, appropriate empiric use of antibiotics, and symptomatic treatment. After the patient’s body temperature returns to normal and blood tests are normal without drainage discharge after oral methylene blue, and chest radiographic examination shows no significant fluid levels, and endoscopy and/or imaging confirms fistula healing, we can consider gradually giving water, liquid diet, and after further verifying the healing, the chest tube can be removed step by step;
(IV) Mediastinal type: the infection and purulent lesions caused by the anastomotic leakage are confined to the mediastinal esophageal bed, without obvious communication with pleural cavities. There can be severe clinical symptoms of systemic infection in contrast to normal bilateral pleural cavities or only reactive pleural effusion. Systemic infection and septic symptoms can usually be severe. Imaging (chest X-ray and/or chest CT) shows normal or just reactive bilateral pleural effusion, and chest CT can reveal effusion or fluid levels near the mediastinal anastomosis, which has diagnostic value. This type of anastomotic leak can be confirmed by endoscopy and/or imaging. In the present cohort, patient number 8 was the mediastinal type. For patients with mediastinal type of anastomotic fistula, depending on the severity of the clinical manifestations, the treatment methods include: conservative treatment (same as the occult type) or concurrent catheter wound abscess endoscopic suction,and thoracotomy drainage in severe systemic infection. Other treatment measures include strict fasting, gastrointestinal decompression, nasogastric enteral nutrition and/or intravenous nutrition, appropriate empiric use of antibiotics, and symptomatic treatment. After the patient’s body temperature returns to normal and blood tests are normal, and chest radiographic examination shows no significant fluid levels in the cavity and mediastinum, and endoscopy and/or imaging confirms fistula healing, we can consider gradually giving water, liquid diet, and after further verifying the healing, the chest tube can be removed step by step;
(V) Hybrid: in some cases of anastomotic leakage, two or more of the left thoracic type, right thoracic type and mediastinal type are presented simultaneously. In the present cohort, patients number 3 and 13 belong to the hybrid type. Based on the clinical severity and infection site, conservative drainage and/or surgical drainage can be used in conjunction with other supportive treatment similar to the above.
It should be noticed that there are no absolute boundaries across the various types above, and each type can convert to another in the natural course of the disease and/or treatment process.
After the occurrence of intrathoracic anastomotic leakage, pleural contamination extent is related to the patient’s prognosis and timing of the diagnosis, which significantly affects the postoperative hospital stay, overall complications, stricture formation, and dysphagia (15,16). Early detection and proper treatment of the intrathoracic anastomotic leakage can reduce the related mortality. Therefore, we should pay attention to the early symptoms of anastomotic leakage, and take positive actions to reduce the serious consequences of anastomotic leakage.
The treatment for anastomotic leakage remains controversial, and the indications for surgical treatment, conservative treatment and endoscopic treatment have not been standardized (17,18). The basic principles of these procedures are the same, which include closing and covering the defect, wrapping the fistula, and draining the contaminated esophagus and mediastinum. The treatment of anastomotic leakage should be based on the degree of contamination of the fistula and the surrounding tissue, as well as the consequent clinical manifestations. The majority of fistulas can be cured by conservative treatment, while a small part of patients require further surgical intervention. When selecting the anastomotic fistula treatment strategy, the clinical manifestations, diagnosis time, etiology, and anastomotic defect size should be taken into account.
The traditional methods for the treatment of anastomotic leakage include adequate drainage, fasting and oral antibiotics, and surgical debridement and drainage in the case of inflammation and mediastinal empyema. The treatment strategy selection should be based on the patient’s clinical manifestations, diagnosis time, etiology, and anastomotic defect size. All such patients should receive intravenous antibiotics, adequate drainage, and nutritional support. Adequate drainage is an important principle in anastomotic leakage treatment, as uncontrolled leakage with inadequate drainage has a mortality rate up to 80% (19).
The majority of fistulas can be cured by conservative treatment, and surgical intervention is rarely used. Methods of surgical intervention include: direct repair of anastomotic fistula or residual stomach; use of the chest wall muscle, omentum, pleural, and pericardial fat tissue for flap reinforcement, and comprehensive restoration surgery including restoration of the gastric remnant with diversion in the digestive tract, and bypass of the distal esophagus end. For patients with uncoated fistula, almost full-circle anastomotic collapse, or the emergence of anastomotic fistula greater than 2 cm along sutures or remnant stomach, esophageal resection is required with esophagus stoma (20,21). Surgical treatment is useful for debridement and drainage, but direct closure of the defect usually fails due to poor organization and local inflammation. Surgical interventions include direct fistula repair, reinforcement flap (chest muscle, omentum, pleural, pericardial fat), bypass surgery (recovery of the stomach into the abdominal cavity, esophagus end stoma) for non-wrapped intrathoracic anastomotic leak with almost entire circumference collapse, or larger fistula along the anastomotic suture or gastric remnant (>2 cm) (7). Because of the high morbidity and mortality rates of second operation, surgical treatment is only recommended for sever symptomatic patients, or non-wrapped intrathoracic anastomotic leak, and those in whom conservative treatment or endoscopic treatment fails.
For early surgical intervention of esophageal anastomotic fistula, Crestanello et al. (22) reported the Mayo Clinic experience, in which the mortality of direct anastomotic fistula repairs was about 15%. Intraoperative thoracic drainage tube placement and endoscopic stent placement may be beneficial for anastomotic leak with small defect (less than 30% of the circumference of the anastomosis). After stent placement, undrained mediastinal contamination and persistent fistula require surgical intervention (7).
In recent years, the endoscopic technique has been reported, especially esophageal stent and suction applied in fistula prevention and treatment (7,23). The main advantage of endoscopic stents is that they immediately close anastomotic defect and reduce mediastinal and pleural contamination, and shorten the oral fasting time, but they are restricted to a fistula anastomotic circumference of no more than 30% and no extensive gastric necrosis. The risk includes further expansion of the anastomotic defect, stent migration, perforation into the trachea, compression of the trachea and heart, bleeding, granulation tissue growth and so on. Currently, the endoscopic treatment is still empirical, and its safety and efficacy needs further evaluation. Despite the use of esophageal stents and wound drainage, undrained mediastinal contamination and sustained fistula still need further intervention.
In conclusion, anastomotic leakage in cardiac carcinoma patients after esophagogastric anastomosis can be classified into five subtypes: occult type, left thoracic type, right thoracic type, mediastinal type, and mixed type. Subtyping of anastomotic leakage is useful and convenient for diagnosis and treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lerut T, Coosemans W, Decker G, et al. Anastomotic complications after esophagectomy. Dig Surg 2002;19:92-8. [PubMed]
- Junemann-Ramirez M, Awan MY, Khan ZM, et al. Anastomotic leakage post-esophagogastrectomy for esophageal carcinoma: retrospective analysis of predictive factors, management and influence on longterm survival in a high volume centre. Eur J Cardiothorac Surg 2005;27:3-7. [PubMed]
- Patil PK, Patel SG, Mistry RC, et al. Cancer of the esophagus: esophagogastric anastomotic leak--a retrospective study of predisposing factors. J Surg Oncol 1992;49:163-7. [PubMed]
- Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg 2004;10:71-5. [PubMed]
- Bardini R, Bonavina L, Asolati M, et al. Single-layered cervical esophageal anastomoses: a prospective study of two suturing techniqu [PubMed]
- Fok M, Ah-Chong AK, Cheng SW, et al. Comparison of a single layer continuous hand-sewn method and circular stapling in 580 oesophageal anastomoses. Br J Surg 1991;78:342-5. [PubMed]
- Schaheen L, Blackmon SH, Nason KS. Optimal approach to the management of intrathoracic esophageal leak following esophagectomy: a systematic review. Am J Surg 2014;208:536-43. [PubMed]
- Bhat MA, Dar MA, Lone GN, et al. Use of pedicled omentum in esophagogastric anastomosis for prevention of anastomotic leak. Ann Thorac Surg 2006;82:1857-62. [PubMed]
- Tang H, Xue L, Hong J, et al. A method for early diagnosis and treatment of intrathoracic esophageal anastomotic leakage: prophylactic placement of a drainage tube adjacent to the anastomosis. J Gastrointest Surg 2012;16:722-7. [PubMed]
- Dai JG, Zhang ZY, Min JX, et al. Wrapping of the omental pedicle flap around esophagogastric anastomosis after esophagectomy for esophageal cancer. Surgery 2011;149:404-10. [PubMed]
- Sepesi B, Swisher SG, Walsh GL, et al. Omental reinforcement of the thoracic esophagogastric anastomosis: an analysis of leak and reintervention rates in patients undergoing planned and salvage esophagectomy. J Thorac Cardiovasc Surg 2012;144:1146-50. [PubMed]
- Yuan Y, Zeng X, Hu Y, et al. Omentoplasty for esophagogastrostomy after esophagectomy. Cochrane Database Syst Rev 2012;11:CD008446. [PubMed]
- Zheng QF, Wang JJ, Ying MG, et al. Omentoplasty in preventing anastomotic leakage of oesophagogastrostomy following radical oesophagectomy with three-field lymphadenectomy. Eur J Cardiothorac Surg 2013;43:274-8. [PubMed]
- Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 2001;88:1157-68. [PubMed]
- Schuchert MJ, Abbas G, Nason KS, et al. Impact of anastomotic leak on outcomes after transhiatal esophagectomy. Surgery 2010;148:831-8; discussion 838-40. [PubMed]
- van der Schaaf M, Lagergren J, Lagergren P. Persisting symptoms after intrathoracic anastomotic leak following oesophagectomy for cancer. Br J Surg 2012;99:95-9. [PubMed]
- Raju GS, Tarcin O. Endoscopic Management of Anastomotic Esophageal Leaks. Tech Gastrointest Endosc 2006;8:66-71.
- Pross M, Manger T, Reinheckel T, et al. Endoscopic treatment of clinically symptomatic leaks of thoracic esophageal anastomoses. Gastrointest Endosc 2000;51:73-6. [PubMed]
- Cassivi SD. Leaks, strictures, and necrosis: a review of anastomotic complications following esophagectomy. Semin Thorac Cardiovasc Surg 2004;16:124-32. [PubMed]
- Martin LW, Hofstetter W, Swisher SG, et al. Management of intrathoracic leaks following esophagectomy. Adv Surg 2006;40:173-90. [PubMed]
- Martin LW, Swisher SG, Hofstetter W, et al. Intrathoracic leaks following esophagectomy are no longer associated with increased mortality. Ann Surg 2005;242:392-9; discussion 399-402. [PubMed]
- Crestanello JA, Deschamps C, Cassivi SD, et al. Selective management of intrathoracic anastomotic leak after esophagectomy. J Thorac Cardiovasc Surg 2005;129:254-60. [PubMed]
- van Boeckel PG, Sijbring A, Vleggaar FP, et al. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther 2011;33:1292-301. [PubMed]


