
Diagnostic usefulness of bronchoscopy for peripheral pulmonary lesions in patients with idiopathic pulmonary fibrosis
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide (1). The International Association for the Study of Lung Cancer took the lead in revising the current 8th TNM staging system for lung cancer, to allow determination of the appropriate treatment and prediction of the prognosis (2). For patients with non-small cell carcinoma, curative treatments such as surgery and radiotherapy have been shown to yield better outcomes in patients with stage I or stage II disease, whereas a multidisciplinary combination (MDD) of surgery, radiotherapy, and drug therapy (i.e., chemotherapy and immunotherapy) is preferred for the treatment of stage III disease, and appropriate drug therapy and palliative care are adopted, depending on the tumor histology and the patient’s general condition for stage IV disease (3,4). In contrast, for patients with small cell carcinoma, surgery is usually performed only in patients with early limited-stage disease without any distant metastases (5).
The prevalence of lung cancer is reported to be high among patients with idiopathic pulmonary fibrosis (IPF), and lung cancers arising in a background of IPF often show higher malignancy grades (6). However, only limited treatment options are available for these patients, due to the high risk of acute exacerbation of IPF (AE-IPF). According to a review article on lung cancer associated with IPF, while surgery is performed in a majority of stage I patients with mild IPF, patients with more advanced malignancy receive chemotherapy or best supportive care, regardless of the severity of IPF (7). Another study reported that during follow-up of patients with interstitial lung disease (ILD), including IPF, tumors need to be identified at an early stage by computed tomography (CT) (8). Thus, early pathological diagnosis of peripheral pulmonary lesions (PPLs) is especially important in patients with IPF, so that lesions that are confirmed as being lung cancer can be treated by curative surgery.
The traditionally used diagnostic techniques for PPLs are bronchoscopy, transthoracic needle biopsy (TTNB), and surgical resection (9). TTNB and surgical resection are superior in terms of the diagnostic yield, but these procedures are also associated with a higher risk of severe complications. A cohort study reported a 9.3% incidence of AE-IPF after surgery for lung cancer, with a 43.9% mortality rate (10). Accordingly, in patients with undiagnosed PPLs, surgery should be avoided, to obviate the unnecessary risk of AE-IPF. In regard to TTNB, although the diagnostic yield is approximately 90%, the pneumothorax rate is about 15%, with at least 7% of the patients requiring chest tube insertion (9). Therefore, TTNB is not the recommended diagnostic tool for patients with IPF, because TTNB, especially for lesions arising in a background of emphysema and/or fibrosis, is associated with a higher risk of pneumothorax (9,11,12).
While the diagnostic yield of traditional semi-blind transbronchial biopsy performed under fluoroscopic guidance was low, ranging from 14% to 63% (13,14), introduction of advanced techniques, such as electromagnetic navigation bronchoscopy, virtual bronchoscopy, radial endobronchial ultrasound (R-EBUS), ultrathin bronchoscopy, and bronchoscopy with the use of a guide sheath (GS), have led to an increase of the diagnostic yield to up to 70% (15). However, reports on the diagnostic outcomes of bronchoscopy for PPLs with a focus on patients with IPF are still scarce. Therefore, we conducted this study to evaluate the diagnostic usefulness of bronchoscopy for PPLs in patients with IPF, and also attempted to identify the factors that could potentially influence the diagnostic yield.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-1067).
Methods
Patients
Data of consecutive patients with IPF who underwent bronchoscopy under R-EBUS guidance for PPLs at our institution between April 2014 and March 2019 were retrospectively reviewed. PPLs were defined as lesions that cannot be directly visualized by bronchoscopy. Among the patients with PPLs, we selected those with IPF, as defined for the purpose of this study as described below.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the National Cancer Center Institutional Review Board (approval No.: 2018–090). The requirement for informed consent was waived due to the retrospective design of the study.
Definition of IPF
IPF is a disease characterized by progressive lung fibrosis and is associated with a poorer prognosis than other ILDs. A recent guideline in 2018 has advocated the use of the following four diagnostic categories based on the patterns observed on high-resolution computed tomographic (HRCT) images: “usual interstitial pneumonia (UIP)”, “probable UIP”, “indeterminate for UIP”, and “alternative diagnosis” (16). IPF can be definitively diagnosed by HRCT alone when the HRCT shows the “UIP” pattern, whereas the histopathological findings should be confirmed by MDD including pathologists when HRCT images show the other three patterns. The “probable UIP” pattern is associated with a greater amount of fibrosis than the “indeterminate for UIP” and “alternative diagnosis” patterns. Thus, the “probable UIP” and “UIP” patterns show similar degrees of fibrosis.
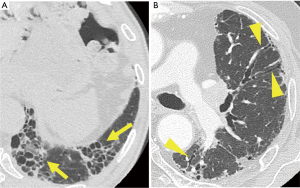
Hence, patients with the HRCT patterns of “UIP” and “probable UIP” were extracted as cases of IPF in this study (Figure 1). The “UIP” pattern is characterized by heterogeneous distribution of honeycombing, with subpleural and basal predominance, with or without peripheral traction bronchiectasis/bronchiolectasis. The “probable UIP” pattern is characterized by reticular abnormalities, also often distributed heterogeneously, showing subpleural and basal predominance, with peripheral traction bronchiectasis/bronchiolectasis; mild ground-glass opacities may be present. We focused on the presence of honeycombing or bronchiectasis, which directly leads to increased branching and bending of the bronchi and disruption of the structure. In addition, we examined the location of the PPL and the background lung, but not its extent.
Outcomes
The bronchoscopic diagnosis was made as follows. Findings of malignancy on histopathology and/or class IV/V lesions on cytology were defined as malignant lesions. Samples with specific benign features, such as inflammation or granuloma, were classified as benign lesions. The final diagnoses in cases of malignancy were based on the histopathological findings of bronchoscopic biopsy or other interventions. Benign lesions were confirmed by histopathology after surgery or based on the findings of follow-up evaluation, such as reduction of the lesion size on follow-up CT. Successful bronchoscopic diagnosis was defined as a match between the bronchoscopic diagnosis and the final diagnosis.
Safety was examined by extracting every complication that could potentially have been related to the procedure.
Variables
The following clinical factors that could potentially influence the diagnostic yield for PPLs or were characteristic of IPF were collected: size, lobe, location, attachment to the costal pleura, bronchus sign, related bronchial generation, association with UIP/probable UIP, and visibility on X-ray. All imaging factors were evaluated on axial HRCT (1 mm or less slice thickness) images obtained within 4 weeks of the bronchoscopy. The images were displayed in a lung window setting (center, −600 Hounsfield units; width, 1,500 Hounsfield units).
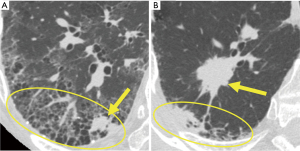
PPLs in a background of IPF are reported to be more likely to occur in the lower lobes and in the lung peripheral regions (17). Therefore, the lobe containing the lesions was classified as “lower” or “others”. In addition, we defined the association of the lesion with the UIP/probable UIP pattern. Cases were classified as “involved” or “not involved” depending on whether the target lesion was inside or outside, respectively, the area of the lung showing the UIP/probable UIP pattern (Figure 2). Moreover, continuous variables were binarized to account for their median values. Accordingly, each factor was divided into the two groups and analyzed; size (small or large; 20.0 mm as threshold), lobe (upper/middle or lower), location in the lung field (inner 2/3 or outer 1/3), pleural attachment (present or absent), bronchus sign (positive or negative), related bronchial generation (≤6 or >6), association with UIP/probable UIP pattern (involved or not involved), visibility on chest X-ray (visible or invisible).
Procedures
The bronchoscopy in all cases was inserted via the mouth using one of the following bronchoscopes [P260F, P290, Y0053 (18), 1T260; Olympus, Tokyo, Japan] along with an R-EBUS probe (UM-S20-17S or UM-S20-20S; Olympus, Tokyo, Japan), under local anesthesia and conscious sedation. GS kits (K-201 or K-203; Olympus, Tokyo, Japan) were used in some cases. After wedging the bronchoscope against the target bronchus, an R-EBUS probe with or without a GS was inserted through the working channel of the bronchoscope. The R-EBUS findings were classified as “within”, “adjacent to”, or “invisible” depending on the relationship between the probe location and the lesion, as previously described (19). After identifying the target, subsequent brushing, forceps biopsy, needle aspiration, and/or cryobiopsy were performed under X-ray fluoroscopic guidance (VersiFlex VISTA; Hitachi, Ltd., Tokyo, Japan).
Statistics
Descriptive statistics are presented in numbers, frequencies (percentages), and median values (ranges). The correlations between the diagnostic yield and clinical factors were statistically analyzed using Fisher’s exact test. Multivariable logistic regression analysis was used to examine the factors that were independently related to the diagnostic yield. Two-tailed P values of <0.05 were considered as denoting statistical significance. A software was used for the statistical analyses (JMP® ver. 14; SAS Institute Inc., Cary, NC, USA).
Results
During the study period, there were 2,744 patients who underwent diagnostic bronchoscopy for PPLs, of whom 94 with IPF. We excluded 2 cases that were lost to follow-up from the analyses. Finally, a total of 92 patients were included in the final analysis; the median (range) age of the patients was 73 (range, 60–90) years, and 78 patients (84.8%) were male. The median (range) size of the target PPLs was 27.1 (range, 11.4–75.3) mm, 74 lesions (80.4%) showed positive bronchus sign, and 78 lesions (84.8%) were involved with UIP/probable UIP pattern.
The overall diagnostic yield was 82.6% (76/92 cases), and the details are summarized in Table 1. No other samplings, in addition to forceps biopsy which was conducted in all cases, were found to have a favorable effect on the diagnostic yield, whereas combined use of rapid on-site cytologic evaluation had a positive effect (diagnostic yield 86.7% vs. 64.7%, P=0.031). Meanwhile, the histopathological diagnoses are shown in Table 2. Two cases in which a definitive diagnosis could not be made (unknown) continue to remain under close follow-up.
Table 1
| Device/technique | Diagnostic cases, n (%) | P value |
|---|---|---|
| Total | 76/92 (82.6) | – |
| Brushing | 0.802 | |
| With | 50/60 (83.3) | |
| Without | 26/32 (81.3) | |
| Needle aspiration | 0.253 | |
| With | 18/24 (75.0) | |
| Without | 58/68 (85.3) | |
| Cryobiopsy | 0.514 | |
| With | 9/10 (90.0) | |
| Without | 67/82 (81.7) | |
| Guide sheath | 0.854 | |
| With | 54/65 (83.1) | |
| Without | 22/27 (81.5) | |
| Virtual bronchoscopy | 0.287 | |
| With | 72/86 (83.7) | |
| Without | 4/6 (66.7) | |
| Rapid on-site cytologic evaluation | 0.031 | |
| With | 65/75 (86.7) | |
| Without | 11/17 (64.7) | |
GS, guide sheath; VBN, virtual bronchoscopy navigation; ROSE, rapid on-site cytologic evaluation.
Table 2
| Diagnosis | Diagnostic, n | Non-diagnostic, n |
|---|---|---|
| Malignant | ||
| Squamous cell carcinoma | 37 | 7 |
| Adenocarcinoma | 25 | 2 |
| Adenosquamous carcinoma | 3 | 1 |
| Non-small cell lung carcinoma | 5 | 0 |
| Small cell lung carcinoma | 3 | 2 |
| Metastatic tumor | 1 | 2 |
| Benign | ||
| Inflammation | 2 | 0 |
| Unknown | 0 | 2 |
A comparison of the influences of clinical factors on the diagnostic yield is shown in Table 3. Multivariable analysis identified the following factors as being associated with a significantly higher diagnostic yield: larger size [P=0.017; odds ratio (OR), 5.33; 95% confidence interval (CI), 1.29–22.01], positive bronchus sign (P=0.035; OR, 4.99; 95% CI, 1.12–22.18), and not involved with UIP/probable UIP pattern (P=0.023; OR and 95% CI, unmeasurable). In addition, when examined separately in association with UIP/probable UIP pattern, the R-EBUS findings were found to significantly affect the diagnostic yield in the involved cases (P<0.001) (Table 4).
Table 3
| Variable | Diagnostic cases, n (%) | Univariable | Multivariable | ||
|---|---|---|---|---|---|
| P value | P value | Odds ratio (95% CI) | |||
| Size† | 0.006 | 0.017 | 5.33 (1.29–22.01) | ||
| Small (≤20.0 mm) | 19/29 (65.5) | ||||
| Large (>20.0 mm) | 57/63 (90.5) | ||||
| Lobe | 0.162 | 0.385 | 1.98 (0.40–9.73) | ||
| Upper/middle | 29/32 (90.6) | ||||
| Lower | 47/60 (78.3) | ||||
| Location | 1.000 | 0.553 | 1.99 (0.21–18.62) | ||
| Inner 2/3 | 12/14 (85.7) | ||||
| Outer 1/3 | 64/78 (82.1) | ||||
| Attachment to the costal pleura | 0.781 | 0.857 | 1.15 (0.25–5.29) | ||
| Present | 50/60 (83.3) | ||||
| Absent | 26/32 (81.3) | ||||
| Bronchus sign | 0.014 | 0.035 | 4.99 (1.12–22.18) | ||
| Positive | 65/74 (87.8) | ||||
| Negative | 11/18 (61.1) | ||||
| Related bronchial generation‡ | 0.380 | 0.966 | 1.03 (0.25–4.30) | ||
| ≤6 | 53/62 (85.5) | ||||
| >6 | 23/30 (76.7) | ||||
| Association with UIP/probable UIP pattern | 0.118 | 0.023 | Unmeasurable | ||
| Involved | 62/78 (79.5) | ||||
| Not involved | 14/14 (100.0) | ||||
| Visibility on chest X-ray | 0.090 | 0.890 | 1.11 (0.25–4.97) | ||
| Visible | 63/73 (86.3) | ||||
| Invisible | 13/19 (68.4) | ||||
†, median [range]: 27.1 [11.4–75.3] mm; ‡, median [range]: 6 [2–12]. CI, confidence interval; UIP, usual interstitial pneumonia.
Table 4
| R-EBUS finding | Involved with UIP/probable UIP pattern | Not involved with UIP/probable UIP pattern | |||
|---|---|---|---|---|---|
| Diagnostic cases, n (%) | P value | Diagnostic cases, n (%) | P value | ||
| Within | 44/47 (93.6) | <0.001 | 10/10 (100.0) | 1.000 | |
| Adjacent to | 17/27 (63.0) | 4/4 (100.0) | |||
| Invisible | 1/4 (25.0) | – | |||
R-EBUS, radial endobronchial ultrasound; UIP, usual interstitial pneumonia.
On the other hand, none of the patients developed respiratory failure necessitating positive pressure ventilation caused by AE-IPF. Although there were 4 cases of bleeding, the bleeding was not serious in any of the cases. Moreover, there was no other complication necessitating intervention or admission, such as pneumothorax or infection.
Discussion
In this study, we investigated the diagnostic usefulness of bronchoscopy for PPLs in IPF patients. To the best of our knowledge, there have been no other reports of such an investigation conducted on a reasonably large number of patients. The diagnostic yield was 82.6%, which was comparable to the rate of 70.6% reported from a meta-analysis of diagnostic bronchoscopy with R-EBUS conducted for PPLs (20).
Multivariable analysis identified a large lesion size, positive bronchus sign, and not involved with UIP/probable UIP pattern as being associated with a significantly higher diagnostic yield. Consistent with these findings, the lesion size and bronchus sign have been reported from previous studies as predictors of the diagnostic yield of bronchoscopy (15,20). In contrast to previous studies, in this study, we investigated the influence of the relationship of PPLs with areas of the lung showing the UIP/probable UIP pattern on the diagnostic yield. We estimated the following reasons for the poor diagnostic yield for PPLs within lung areas showing the UIP/probable UIP pattern.
First, when the target lesion is within an area of the lung showing changes of IPF, it is often difficult to identify the bronchus leading to the lesion. The pathology of UIP is characterized by inflammation and repair, resulting in remodeling and dense fibrosis of the lungs and peripheral airways (16). This causes the bronchi to be pulled and dilated, resulting in bent bronchi, which do not show normal branching on CT. The lung parenchyma also becomes fibrotic and cystic, making it difficult to distinguish between bronchi and cysts. Furthermore, pulmonary fibrosis and pulmonary emphysema are similar with respect to destruction of the architecture of the lung parenchyma. The diagnostic yield of bronchoscopy for PPLs in patients with severe emphysema has been reported to be worse than that in patients with normal or mild emphysema (21). This is because in patients with advanced emphysema, the destruction of the lung parenchyma makes it difficult to detect the bronchus sign, and the same may be true in patients with IPF. In fact, when we examined the diagnostic yield of each R-EBUS finding, which was strongly related to the presence/absence of the bronchus sign, separately according to the location of the lesion relative to areas of the lung showing the UIP/probable UIP pattern, we found significant differences in the diagnostic yield depending on the R-EBUS findings in the involved cases, but not in the not involved cases (Table 4). This may be due to the fact that it was relatively difficult to identify the orientation of the bronchus towards the lesion by R-EBUS in the involved cases, as the boundary of the lesion with the background lung was obscured in these cases.
Second, target lesions within lung areas with IPF might be difficult to detect by X-ray fluoroscopy. It has been reported that lung cancers associated with IPF are often centered in fibrotic areas of the lung (8,17), and lesions overlapping the reticular shadows of IPF often cannot be visualized on a chest X-ray (22). In addition, most PPLs in patients with IPF are found in the lower lobe (17), sometimes in a position hidden by the diaphragm. We show a representative case of a PPL with IPF that was invisible on X-ray fluoroscopy (Figure 3). Although there was no statistically significant difference in the visibility on chest X-ray, lesions within areas of IPF, as in this case, can often not be visualized on chest X-ray or X-ray fluoroscopy, which may explain the lower diagnostic yield.
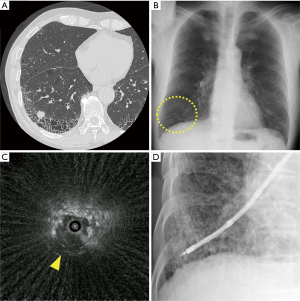
On the other hand, the R-EBUS findings of IPF might be different from those of the normal parenchyma, as the lung structure is destroyed and replaced by fibrosis. The difference in the findings of R-EBUS between normal lung parenchyma and honeycomb lung has been investigated in autopsy lungs (23). Normal lungs showed relatively regular and fine granular hyperechoic patterns, whereas honeycomb lungs exhibited a gross patchy combination of hyperechoic and hypoechoic patterns. In fact, as shown in Figure 3C, the R-EBUS findings of lesions involved in IPF were relatively difficult to identify. Nevertheless, the diagnostic yield was the highest when the R-EBUS probe was within the lesion, consistent with previous reports (15,20) (Table 4). Although the R-EBUS findings may vary somewhat, the sufficient detection of target PPLs (i.e., within the lesion) could improve the diagnostic yield, even in patients with IPF.
In terms of complications, there were no cases of pneumothorax requiring treatment in this study, whereas a previous meta-analysis reported an incidence rate of pneumothorax of 1.5% (15). The possibility of sampling at the location of the lesion identified by R-EBUS is thought to account for the safety of the procedure. On the other hand, the diagnostic yield was low in cases where the lesions could not be identified clearly by R-EBUS or chest X-ray, which could possibly be related to the fact that sampling was not enforced in these cases. In addition, there was no case of AE-IPF. Although there are no reports of the risk of AE-IPF when bronchoscopy is performed for the diagnosis of PPLs in cases of IPF, a certain degree of risk has been reported when bronchoscopy is performed for the diagnosis of ILD (24). Thus, our results suggest that bronchoscopy for PPLs in patients with IPF can be performed safely without serious complications.
The present study had several limitations. First, it was a retrospective, non-randomized study conducted at a single cancer institution. Although we enrolled consecutive cases, there might be some bias in the selection of the study subjects. In fact, most cases were diagnosed as having malignant tumors, which may have contributed to the high diagnostic yield (20,25). Second, we did not compare the diagnostic yield between patients with and without IPF. Third, selection of the bronchoscopes and devices to be used were left to the discretion of each operator. In this retrospective setting, we could not determine if the differences in the scopes/devices selected might have influenced the results. Therefore, a prospective study is warranted for further clarification of the findings.
Conclusions
Bronchoscopy using R-EBUS is safe and provides an acceptable diagnostic yield for PPLs, even in patients with IPF.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-1067
Data Sharing Statement: Available at
Peer Review File: Available at
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-1067). YM reports grants from National Cancer Center Research and Development Fund, grants from Grant-in-Aid for Scientific Research on Innovative Areas, grants from Hitachi, Ltd., personal fees from Olympus, personal fees from Erbe Elektromedizin GmbH, personal fees from AMCO Inc., outside the submitted work. TT reports grants from Japan Agency for Medical Research and Development, grants from Foundation for Promotion Cancer Research, personal fees from Nippon Medical School Foundation, personal fees from Hamamatsu University School of Medicine, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013). This human study was approved by the National Cancer Center Institutional Review Board (approval: No. 2018–090). The requirement for informed consent was waived due to the retrospective design of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-237. Erratum in: Ann Oncol 2019;30:863-70 doi: 10.1093/annonc/mdy474. [Crossref]
- Früh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi99-105. [Crossref] [PubMed]
- Raghu G, Amatto VC, Behr J, et al. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J 2015;46:1113-30. [Crossref] [PubMed]
- Tomassetti S, Gurioli C, Ryu JH, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015;147:157-64. [Crossref] [PubMed]
- Yoshida R, Arakawa H, Kaji Y. Lung cancer in chronic interstitial pneumonia: early manifestation from serial CT observations. AJR Am J Roentgenol 2012;199:85-90. [Crossref] [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S.
- Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014;147:1604-1611.e3. [Crossref] [PubMed]
- Huang CT, Ruan SY, Liao WY, et al. Risk factors of pneumothorax after endobronchial ultrasound-guided transbronchial biopsy for peripheral lung lesions. PLoS One 2012;7:e49125 [Crossref] [PubMed]
- Bai C, Choi CM, Chu CM, et al. Evaluation of Pulmonary Nodules: Clinical Practice Consensus Guidelines for Asia. Chest 2016;150:877-93. [Crossref] [PubMed]
- Rivera MP, Mehta AC; American College of Chest Physicians. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:131S-48S.
- Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000;117:1049-54. [Crossref] [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Oh SY, Kim MY, Kim JE, et al. Evolving Early Lung Cancers Detected During Follow-Up of Idiopathic Interstitial Pneumonia: Serial CT Features. AJR Am J Roentgenol 2015;204:1190-6. [Crossref] [PubMed]
- Sasada S, Izumo T, Chavez C, et al. A new middle-range diameter bronchoscope with large channel for transbronchial sampling of peripheral pulmonary lesions. Jpn J Clin Oncol 2014;44:826-34. [Crossref] [PubMed]
- Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. [Crossref] [PubMed]
- Ali MS, Trick W, Mba BI, et al. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirology 2017;22:443-53. [Crossref] [PubMed]
- Lee KM, Lee G, Kim A, et al. Clinical outcomes of radial probe endobronchial ultrasound using a guide sheath for diagnosis of peripheral lung lesions in patients with pulmonary emphysema. Respir Res 2019;20:177. [Crossref] [PubMed]
- Kishi K, Homma S, Kurosaki A, et al. High-resolution computed tomography findings of lung cancer associated with idiopathic pulmonary fibrosis. J Comput Assist Tomogr 2006;30:95-9. [Crossref] [PubMed]
- Omori S, Takiguchi Y, Hiroshima K, et al. Peripheral pulmonary diseases: evaluation with endobronchial US initial experience. Radiology 2002;224:603-8. [Crossref] [PubMed]
- Amundson WH, Racila E, Allen T, et al. Acute exacerbation of interstitial lung disease after procedures. Respir Med 2019;150:30-7. [Crossref] [PubMed]
- Dhillon SS, Harris K. Bronchoscopy for the diagnosis of peripheral lung lesions. J Thorac Dis 2017;9:S1047-58. [Crossref] [PubMed]


