Clinical role of a new prognostic score using platelet-to-lymphocyte ratio in patients with malignant pleural mesothelioma undergoing extrapleural pneumonectomy
Introduction
Malignant pleural mesothelioma (MPM) is a highly aggressive and refractory thoracic malignancy with extremely poor prognosis (1). The trimodality approach including induction chemotherapy, extrapleural pneumonectomy (EPP) and radiation therapy has been increasingly accepted in selected patients over the past two decades (2,3). However, recent publication of the mesothelioma and radical surgery (MARS I) trial has diminished the role of this surgical procedure in the treatment of MPM for its invasiveness and poor short-term outcome (4). Alternatively, another surgical procedure, pleurectomy/decortication (P/D) has been focused as a less invasive procedure (5).
Because of the high morbidity of EPP, patient selection is crucial to potentially improve treatment outcome. Although attempts to establish prognostic scoring models using several clinicopathologic factors were performed, those scoring system were complicated and rarely used except in the specific context of clinical trials (6,7). Therefore, a simple, easy to calculate prognostic score able to stratify patients according to prognosis prior to treatment is highly desired.
Asbestos induced chronic inflammation plays a key role in the pathogenesis of MPM. Recently, several biomarkers reflecting systemic inflammation have been shown as independent prognostic factors for patients with MPM who received systemic therapy or surgery (8-12). A recent report from the International Association for the Study of Lung Cancer (IASLC) Staging Committee using an international large database has identified that platelet count and white blood cell (WBC) count are independent prognostic factors in addition to histology, sex and age (8). Pinato et al. reported that neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) could be externally validated prognostic indices in patients with MPM (9). These simple biomarkers reflecting systemic inflammation were easily obtained before starting treatments. Therefore, combination of these biomarkers and clinicopathological factors could be used to appropriately stratify patients who could receive the greatest benefits from EPP.
In the current study, we hypothesize that inflammation related biomarkers can be used to establish the new scoring system in patients undergoing EPP. Furthermore, we verified the established scoring system in an independent validation cohort who underwent EPP for MPM in a different country.
Materials and methods
The ethics committee of three institutes including University of Toronto, Kyushu University and National Kyushu Cancer Center approved this study and granted a waiver for patient consent.
Patient population and treatment protocol in the training cohort
Of 85 patients who underwent EPP for MPM during January 2001 to April 2011 at Toronto General Hospital, University of Toronto, 65 patients whose blood test results before initial therapy were available were retrospectively analyzed as a training cohort. All patients were histologically proven diagnosis of MPM. The preoperative workup and treatment protocol including chemotherapy, EPP and postoperative radiation therapy were described previously (13). Briefly, the patients underwent mostly two to three cycles of platinum based induction chemotherapy. The doublet cisplatin-vinorelbine was used preferentially between 2001 and 2003 (n=13) and was then switched to cisplatin-pemetrexed (n=29) or cisplatin-raltitrexed (n=4) since 2004. EPP which consists of en bloc resection including the lung, parietal pleura, ipsilateral diaphragm, and ipsilateral pericardium was performed 3 to 6 weeks after the completion of chemotherapy. Postoperative hemithoracic radiotherapy started 6 to 12 weeks after EPP. From 2008, induction radiotherapy protocol which consists in 25 Gy (5 Gy in 5 daily fractions) of radiation administered to the entire ipsilateral hemithorax by intensity-modulated radiotherapy technique (IMRT) was started (14). Patients with induction radiotherapy underwent EPP within 2 weeks after the end of radiotherapy. Patients were staged according to International Mesothelioma Interest Group guidelines after EPP as pathological stage. In terms of histological subtype, we used the histological diagnosis from diagnostic pleural biopsy when there was a discrepancy between the diagnosis from the biopsy sample and that from EPP sample to eliminate the influence of induction chemotherapy. Baseline full blood count including hemoglobin, absolute platelet count, WBC count, and its different counts was obtained from blood test performed before any intervention or treatment. The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count. The PLR calculated by dividing the absolute platelet count by the absolute lymphocyte count.
Patient population and treatment protocol in the validation set
Thirty-two patients who underwent EPP for MPM from January 2001 to July 2014 at Kyushu University (n=10) and Kyushu Cancer Center (n=22), Japan were retrospectively analyzed. The multimodal treatment protocols for patients with MPM in these two institutes were essentially the same and were described previously (15-17). Briefly, patients underwent EPP with preoperative or postoperative platinum based chemotherapy and/or radiotherapy. Previously, 2 to 3 cycles of platinum based induction triplet chemotherapy consisting of cisplatin, gemcitabine and vinorelbine every 4 weeks was used preferentially from 2001 to 2006 (n=10) and it was then switched to platinum based doublet chemotherapy consisting of cisplatin and pemetrexed, every 3 weeks in 2006 (n=12).
Statistical analysis
To determine the optimal cutoff values of age and each hematological parameter, receiver operating characteristic (ROC) curves were conducted and the Youden index was used to maximize the sum of sensitivity and specificity. The prognostic value of each hematological parameter was assessed by comparing the area under ROC curves (AUC) of each parameter using a non-parametric method.
Survival was calculated from the date of the initial treatment until death due to any cause or the last follow-up (censored). The survival curves were constructed based on the Kaplan-Meier method. The prognostic value of the variables was examined using univariate Cox regression with categorical variables. The prognostic value of each factor was subsequently explored on multivariate analysis using a Cox proportional hazard model. The survival curves of risk groups were determined by the Kaplan-Meier method and compared with the log-rank test. Statistical significance was defined as P less than 0.05. All data were analyzed using JMP, version 5.0 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient demographics in the training and the validation cohort
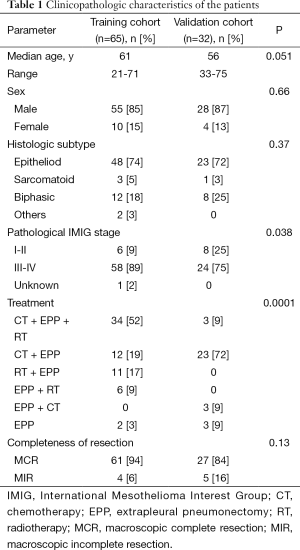
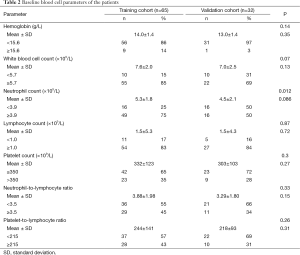
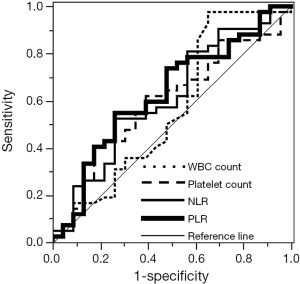
The patient characteristics in both training and validation cohort are shown in Table 1. There were no significant differences in age, sex and histology between the cohorts. However, patients in the training cohort had a significantly more advanced pathological IMIG stage than patients in the validation cohort. In addition, there was significant difference in treatment modalities between the cohorts. In the training cohort, thirty-four patients (52%) underwent trimodality therapy consisting of induction chemotherapy, EPP and radiotherapy, 11 patients (17%) underwent induction radiotherapy followed by EPP, while in the validation cohort, only 3 patients (9%) underwent trimodality therapy and the majority of the patients (n=23, 72%) underwent induction chemotherapy followed by EPP. The most frequently used regimen in the induction chemotherapy was cisplatin and pemetrexed in the two cohorts (training cohort: 63%, validation cohort: 55%). The baseline hematologic parameters in both training and validation cohorts are shown in Table 2. No significant difference was observed in the mean values of any hematologic parameter between the cohorts. The cutoff values of each parameter were determined by ROC curve for 2-year survival and Youden index in the training cohort (Figure 1). AUC with 95% CI of each parameter in the training cohort are as follows: hemoglobin, 0.587 (95% CI, 0.429-0.729); WBC count, 0.565 (95% CI, 0.402-0.715); neutrophil count, 0.580 (95% CI, 0.415-0.729); lymphocyte count, 0.580 (95% CI, 0.434-0.713); platelet count, 0.594 (95% CI, 0.445-0.728); NLR, 0.611 (95% CI, 0.457-0.746); and PLR, 0.628 (95% CI, 0.476-0.759). PLR showed the highest AUC among the parameters although no statistically significant difference was observed between AUC of each parameter. When NLR and PLR were compared with linear regression, there was a strong correlation between the factors (r=0.6793, P<0.0001).
Full table
Full table
Univariate and multivariate analysis for prognostic factors in the training cohort
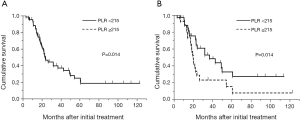
The median overall survival time of all patients in the training cohort was 23.7 months with a median follow-up time of 29.5 months (range, 3.0-123.1 months). The overall 2- and 5-year survival was 48.7% and 21.7%, respectively (Figure 2A). In univariate analyses in the training cohort, female gender (P=0.0019), epithelioid histological subtype (P=0.048), pathological stage I-II (P=0.025), WBC count <5.7×109/liter (P=0.0028), neutrophil count <3.9×109/liter (P=0.018), platelet count <350×109/liter (P=0.005), NLR <3.5 (P=0.041), and PLR <215 (P=0.030) were predictors of favorable survival (Table 3). There was no survival difference between treatment groups in the training cohort. Multivariate analysis in the training cohort using the Cox proportional hazards regression model confirmed that sex (hazard ratio, 0.20; 95% CI, 0.032-0.66; P=0.0053) and PLR (hazard ratio, 0.5; 95% CI, 0.24-0.99; P=0.049) as independent predictors of overall survival. When NLR was analyzed in multivariate analysis instead of PLR, it was not an independent predictor of overall survival (hazard ratio, 1.58; 95% CI, 0.82-3.1, P=0.1734). Platelet count was also an independent prognostic factor for overall survival when it was put into multivariate analysis instead of PLR (hazard ratio, 0.45; 95% CI, 0.22-0.91, P=0.027). Median overall survival in the training cohort stratified by PLR was 37.0 and 20.0 months for PLR <215 (n=37) and ≥215 (n=28), respectively (P=0.014) (Figure 2B).

Full table
Establishing a new prognostic scoring system
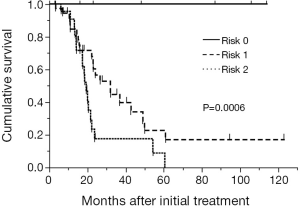
A scoring system based on the results of the ROC analysis for survival and multivariate analysis in the training cohort was developed. The prognostic scoring system was established using sex and PLR. There was no difference of PLR between sex (male vs. female =248±45 vs. 243±19, P=0.92). The score was defined as follows; female:male =0:1 point; PLR <215:≥215=0:1 point. The patients were classified into three risk groups according to the sum of scores: risk 0 (0 point) in 6 patients (9.2%), risk 1 (1 point) in 36 patients (55.4%), and risk 2 (2 points) in 23 patients (35.4%). Median survival time of these three groups were not reached, 32.0 months and 19.4 months for risk 0 (n=6), 1 (n=36) and 2 (n=23), respectively (P=0.0006, Figure 3).
External validation of the scoring system
The established prognostic scoring system was externally validated in a cohort of 32 patients who underwent EPP for MPM during January 2001 to July 2014 at two institutes in Japan. The median overall survival time was 37.4 months with a median follow-up time of 41 months (range, 2-157 months) (Figure 4A). The overall 2- and 5-year survival was 74.6% and 26.9%, respectively. The median overall survival time according to the risk group was not reached, 45.9 and 14.5 months for risk 0 (n=4, 12.5%), 1 (n=18, 56.3%) and 2 (n=10, 31.3%), respectively (P=0.0002) (Figure 4B).

Discussion
In the present study, we showed that PLR is an independent prognostic factor in the patients with MPM who underwent EPP and established the new scoring system using PLR which is simple and useful to predict outcome after EPP. The prognostic scoring system is externally validated using two independent cohorts in another country.
Systemic inflammation has been considered to contribute to multiple stages of cancer progression. Peripheral blood test before starting treatment may reflect inflammatory status of the patient with malignancy including MPM. Pass et al. has recently reported that elevated WBC count and platelet count were predictive of outcome in addition to histologic subtype, age and sex in the situation before any staging procedure to evaluate the patient for surgery (8). While elevated neutrophil and platelet count reflect systemic inflammation, decreased lymphocyte count is associated with immunosuppression. Therefore, NLR and PLR which combine both inflammatory and immunosuppressive indices might be better biomarkers than WBC count or platelet count. In our study, low WBC count, low neutrophil count, low platelet count, low NLR, and low PLR were predictors of favorable survival in univariate analysis. High lymphocyte count showed a tendency of better prognosis. Among these parameters, PLR showed the highest AUC for survival. Therefore, PLR may predict outcome more reliably than the other parameters. In multivariate analysis, low PLR remained an independent favorable prognostic factor in addition to sex. Although low platelet count was also an independent predictor of survival when it was analyzed instead of PLR, these results suggest that PLR may be a more consistent, reliable marker than NLR or absolute platelet count.
EPP is the most aggressive surgical strategy in the treatment of MPM and has been performed widely in the last two decades. We and others have reported the feasibility and survival benefit of EPP in the setting of multimodal treatment for selected patients (18-21). Another surgical method, P/D has recently attracted attention for its lower invasiveness although the surgical technique of P/D has not been fully standardized yet. Cao et al. reported the meta-analysis of surgical treatments for MPM comparing perioperative and long-term outcomes of EPP and extended P/D. They showed that selected patients who underwent extended P/D had lower mortality with similar long-term outcome compared to EPP (22). However, since there has been no randomized trial comparing EPP and P/D, it remains controversial as to which surgical procedure offers the best rates of survival. In our previous study, favorable survival could be achieved by EPP with median survival of 59 months in highly selected patients with negative mediastinal nodes who completed the trimodality treatment protocol (13). In order to adequately select the patients who could receive the full benefit from this invasive treatment, we need clinical indices that could predict outcome before starting the treatment.
Our new prognostic scoring system consists of two parameters; gender and PLR. These two indices are simple, objective and easy to obtain at the time of diagnosis. Three risk groups based on the sum of the points of each index predict the prognosis of the patients clearly in the training cohort. The scoring system was externally validated with another patient cohort in a different country. The treatment strategies in the training and validation cohorts were extremely different. Half of the patient in the training cohort underwent trimodality therapy with induction chemotherapy and adjuvant hemithoracic radiotherapy, while most of the patient in the validation cohort underwent only induction chemotherapy. In addition, the training cohort included patients who underwent our new treatment protocol with induction radiotherapy. However, there was no survival difference between the treatment groups in both the training and the validation cohorts. In the validation cohort, the scoring system was well validated in terms of outcome although the patient number was very small and the follow-up period was very small, especially in the risk 0 group. The distribution of the patients in the three risk groups was very similar between cohorts (risk 0, 1, 2; 9.2%, 55%, 35% in the training cohort vs. 13%, 56%, 31% in the validation cohort, respectively). This simple scoring system may therefore be worth validating in larger cohorts.
There are some limitations in our study. Our study is retrospective and included a small number of patients who underwent EPP over 10 years. The results of blood test before starting induction chemotherapy were only available in 76.5% of patients in the training cohort. The treatment modalities in the training cohort had great heterogeneity and were significantly different from those in the validation cohort. In addition, patients in the training cohort had a significantly more advanced pathological stage than those in the validation cohort. Therefore, our findings should be validated in larger independent series. We also need to apply this scoring system in a prospective fashion not only for patients undergoing EPP but also for all patients presenting with MPM and those undergoing P/D.
In conclusion, we showed that PLR could be a useful biomarker for patients with MPM. The established new prognostic scoring system using PLR is simple and useful for predicting the prognosis of patients with MPM undergoing EPP. Further study should be done to examine the role of this scoring system to optimize treatment strategy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005;353:1591-603. [PubMed]
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63; discussion 63-5. [PubMed]
- Flores RM, Krug LM, Rosenzweig KE, et al. Induction chemotherapy, extrapleural pneumonectomy, and postoperative high-dose radiotherapy for locally advanced malignant pleural mesothelioma: a phase II trial. J Thorac Oncol 2006;1:289-95. [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [PubMed]
- Nakas A, Trousse DS, Martin-Ucar AE, et al. Open lung-sparing surgery for malignant pleural mesothelioma: the benefits of a radical approach within multimodality therapy. Eur J Cardiothorac Surg 2008;34:886-91. [PubMed]
- Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998;16:145-52. [PubMed]
- Fennell DA, Parmar A, Shamash J, et al. Statistical validation of the EORTC prognostic model for malignant pleural mesothelioma based on three consecutive phase II trials. J Clin Oncol 2005;23:184-9. [PubMed]
- Pass HI, Giroux D, Kennedy C, et al. Supplementary prognostic variables for pleural mesothelioma: a report from the IASLC staging committee. J Thorac Oncol 2014;9:856-64. [PubMed]
- Pinato DJ, Mauri FA, Ramakrishnan R, et al. Inflammation-based prognostic indices in malignant pleural mesothelioma. J Thorac Oncol 2012;7:587-94. [PubMed]
- Kao SC, Pavlakis N, Harvie R, et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res 2010;16:5805-13. [PubMed]
- Kao SC, Klebe S, Henderson DW, et al. Low calretinin expression and high neutrophil-to-lymphocyte ratio are poor prognostic factors in patients with malignant mesothelioma undergoing extrapleural pneumonectomy. J Thorac Oncol 2011;6:1923-9. [PubMed]
- Kao SC, Vardy J, Chatfield M, et al. Validation of prognostic factors in malignant pleural mesothelioma: a retrospective analysis of data from patients seeking compensation from the New South Wales Dust Diseases Board. Clin Lung Cancer 2013;14:70-7. [PubMed]
- de Perrot M, Feld R, Cho BC, et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:1413-8. [PubMed]
- Cho BC, Feld R, Leighl N, et al. A feasibility study evaluating Surgery for Mesothelioma After Radiation Therapy: the "SMART" approach for resectable malignant pleural mesothelioma. J Thorac Oncol 2014;9:397-402. [PubMed]
- Yoshino I, Yamaguchi M, Okamoto T, et al. Multimodal treatment for resectable epithelial type malignant pleural mesothelioma. World J Surg Oncol 2004;2:11. [PubMed]
- Maruyama R, Shoji F, Okamoto T, et al. Triplet chemotherapy with cisplatin, gemcitabine and vinorelbine for malignant pleural mesothelioma. Jpn J Clin Oncol 2005;35:433-8. [PubMed]
- Okamoto T, Yano T, Haro A, et al. Treatment for recurrence after extrapleural pneumonectomy for malignant pleural mesothelioma: A single institution experience. Thoracic Cancer 2013;4:66-70.
- Weder W, Kestenholz P, Taverna C, et al. Neoadjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. J Clin Oncol 2004;22:3451-7. [PubMed]
- Rice DC, Stevens CW, Correa AM, et al. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg 2007;84:1685-92; discussion 1692-3.
- Schipper PH, Nichols FC, Thomse KM, et al. Malignant pleural mesothelioma: surgical management in 285 patients. Ann Thorac Surg 2008;85:257-64. [PubMed]
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13. [PubMed]
- Cao CQ, Yan TD, Bannon PG, et al. A systematic review of extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Oncol 2010;5:1692-703. [PubMed]


