The total artificial heart
Mechanical circulatory support, including the total artificial heart (TAH) and its more widely used counterpart the left ventricular assist device (LVAD), has a vital and expanding role in the care of patients with end-stage heart failure. Worldwide, the prevalence of heart failure is increasing. The Global Burden of Disease Study 2013 demonstrated that the number of cardiovascular deaths has increased with the aging population and accounts for approximately one-third of all deaths (1). Although considered to be the gold standard for the treatment of end stage heart failure, heart transplantation is only able to meet a small subset of the clinical need. The number of heart transplants performed worldwide has remained fixed at 4,000 to 4,500 per year for the last decade (2). Advances in durable mechanical circulatory support have thus seen an exponential growth in use (3). In 2013, the number of durable device implants [2,744] exceeded the number of heart transplants [2,614] in North America. Roughly forty percent of patients receiving heart transplantation are bridged with mechanical circulatory support (2). The vast majority of patients with end stage heart failure can be adequately treated with isolated LVAD support. However, there is a small subset of patients with profound biventricular dysfunction or other severe structural abnormalities that is at high risk for poor outcomes following LVAD implantation. The TAH is an important therapeutic option for such patients (4). This review paper will focus on the history, indications, surgical implantation, post device management, outcomes, complications, and future direction of the TAH.
History of the TAH
The development of TAHs was preceded by decades of research, experimentation, and collaboration conducted at various institutions around the world.
Much of the developmental work leading to the modern TAH was performed by Willem Kolf and his trainees. In 1957, Dr. Kolff and Tetsuzo Akutsu performed their first successful animal TAH implant in a dog supporting circulation for 90 min (5).
In 1967, the first human heart transplant was performed in South Africa by Christiaan Barnard (6). However, early experience with poor post-transplant survival would soon dampen enthusiasm. A similar experience would be repeated with the artificial heart.
In 1969, Denton Cooley and Domingo Liotta performed the first human TAH implant using the Liotta Heart (an experimental device designed by Dr. Liotta, a former trainee of Dr. Kolff) (7). The patient was a 47-year-old man with ischemic cardiomyopathy who was unable to come off cardiopulmonary bypass following remodeling ventriculoplasty. The TAH successfully provided hemodynamic support, but the patient quickly developed hemolysis and progressive renal failure. The patient was bridged to transplantation after 64 h of support but unfortunately died 32 h later from pseudomonal sepsis. The ground breaking event was filled with controversy regarding improper consent and experimentation (8).
The following decade was notable for advances in immune suppression and improved outcomes for heart transplantation. Following the introduction of cyclosporine, Norman Shumway and the Stanford group reported an improvement in 1 year survival from 63% in 1980 to 83% in 1985 (9). It would take longer for similar success with the TAH.
In 1981, Dr. Cooley performed the second human TAH implant (the Akutsu III, developed by Dr. Akutsu) in a 36-year-old man with post cardiotomy shock following coronary bypass surgery (10). The postoperative course was notable for renal failure and severe hypoxia secondary to left pulmonary venous obstruction and required veno-venous extracorporeal membrane oxygenation. The patient was bridged to transplantation after 55 h of support, but unfortunately died 1 week later from overwhelming sepsis.
Due to the poor outcomes, it was felt that further human TAH implants should be restricted to patients who were not candidates for transplantation and had no other alternatives. In 1982, William DeVries performed the well-publicized permanent (now referred to as destination therapy) TAH implant of an artificial heart (the Jarvik 7, designed by Robert Jarvik, another Kolff trainee) into Dr. Barney Clark (11). Dr. Clark was a 61-year-old man with non-ischemic cardiomyopathy, refractory ventricular arrhythmias and multiorgan dysfunction that precluded consideration for transplant. He was hemodynamically supported for 112 days. His postoperative course was difficult and notable for respiratory failure requiring tracheostomy and resection of pulmonary blebs, fracture of the prosthetic mitral valve strut requiring replacement of the artificial left ventricle, fevers, stroke, seizures, delirium, renal failure, and bleeding related to anticoagulation. He ultimately succumbed to pseudomembranous colitis. Despite his willingness to volunteer for the benefit of science, the public spectacle of his story provoked discussions of Frankenstein and the ethics of extreme human experimentation.
As transplant outcomes continued to improve, further TAH implants as destination therapy was abandoned and attention refocused on bridging patients to transplantation. The majority of this focused on development of LVADs, however work on the TAH continued for the subset in whom LVADs were not adequate.
In 1985, Copeland successfully implanted the Jarvik 7 TAH as a bridge to transplantation (12). The recipient was a 25-year-old man with non-ischemic cardiomyopathy listed for transplantation but deteriorating with refractory ventricular tachycardia. With device support, he had marked hemodynamic improvement, but developed a stroke after 7 days of support. He had neurologic improvement and underwent transplantation 2 days later. Findings at the time of his surgery were notable for fibrinous deposits in the mechanical valve housings of the device. He subsequently had full neurologic recovery and did well with his transplant. He died 5.5 years later from lymphoma (13).
As success with the TAH as a bridge to transplant accumulated a trial of the CardioWest TAH (developed from the Jarvik 70 and now marketed as the Syncardia TAH) was started in 1993 and completed in 2002 (14). Eighty-one patients were implanted under the trial protocol with 79% survival to transplantation and an overall 1 year survival of 70%.
Development of the portable Freedom driver (both CE Mark and FDA approved) has enabled discharge of artificial heart patients while they awaited transplant (see Figure 1). With increased clinical success, there has been increased utilization and to date there has been more than 1,400 Syncardia/CardioWest TAH implants (15). In contrast, there have been more than 20,000 Heartmate II LVAD implants (16).
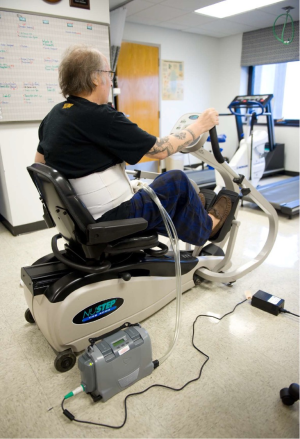
Patient selection
Compared with LVADs, the TAH is implanted in a much smaller subset of patients. The Syncardia TAH (SynCardia Systems, Inc., Tucson, AZ) is the only commercially available TAH in the United States approved by the Food and Drug Administration (FDA) (17) (see Figure 2).
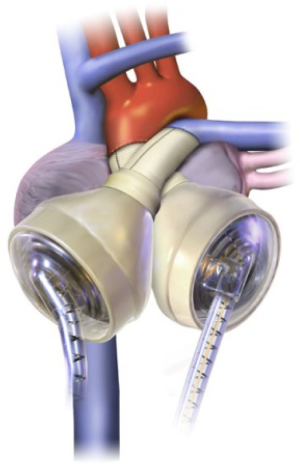
The pivotal clinical trial published in 2004, included patients with irreversible biventricular cardiac failure (14). The device is indicated in patients who are eligible for transplantation with New York Heart Association Class IV symptoms with the appropriate chest size (BSA 1.7-2.5 m2 or >10 cm between the 10th thoracic vertebrae and the sternum) who have hemodynamic insufficiency requiring cardioactive medications (vasopressors and inotropes) or mechanical support.
Importantly, patients were only considered if they were deemed to not be LVAD candidates. These patients were unable to wean from cardiopulmonary bypass, had central venous pressures greater than 18 mmHg, RV ejection fractions less than 20%, ventricular tachycardia, aortic prostheses, or RV damage at time of sternotomy. Thus, based on the original clinical trials, TAH’s primary role is in patients dying from biventricular failure who are not LVAD candidates as a salvage therapy to bridge to heart transplantation.
As experience with TAH advances, growing evidence supports its use in patients with biventricular heart failure. Patients with concurrent right ventricular failure in addition to left ventricular failure have poorer outcomes with LVADs than patients with isolated left ventricular failure (18,19). In these patients, biventricular support is indicated. While the orthotopic TAH provides definitive biventricular support, paracorporeal BiVADs provide an alternative MCS strategy for biventricular dysfunction. This includes temporary and durable RVADs in conjunction with an LVAD. Initial biventricular mechanical support for critically ill patients usually provides higher cardiac output at lower doses of inotropes which can help resuscitate end organ malperfusion. While no head-to-head prospective randomized controlled trials have compared these two types of mechanical circulatory support, one small retrospective study showed no difference in mortality for patients implanted with a TAH compared with BiVADs (20). Conversely, although the number of implants remains too small to draw conclusions, analysis of the INTERMACS registry has suggested improved short term survival of patients implanted with a TAH compared to BiVADs (3).
Identifying patients with biventricular heart failure who would require biventricular support is challenging. A number of risk scores have been devised to identify these patients pre-operatively. For example, Drakos et al. statistically analyzed retrospective outcomes data for patients who underwent LVAD placement to determine pre-implant characteristics which were predictive of RV failure post-implant (21). Their scoring system includes six categories: destination therapy as device indication, use of intra-aortic balloon pump, elevated pulmonary vascular resistance, inotrope dependency, obesity, and use of ACE inhibitor or ARB. Higher scores equate to higher rates of RV dysfunction and higher mortality post-operatively. Another scoring system which does not use direct hemodynamic measurements includes vasopressor requirements, elevated aspartate aminotransferase, elevated bilirubin, and elevated creatinine to predict RV dysfunction (22).
The TAH is also indicated in end stage heart failure patients with anatomical or other clinical conditions that are not well treated with LVADS. This includes patients with small/non dilated ventricles (4) (hypertrophic, infiltrative, and other restrictive cardiomyopathies) and patients requiring significant concomitant repair [e.g., post-infarct ventricular septal defects, aortic root/ascending aortic aneurysms, congenital heart disease (23), massive LV thrombus].
Surgical implantation
The CardioWest TAH (SynCardia Systems, Inc., Tucson, AZ) is currently the only commercially available TAH in the United States approved by the FDA as a bridge to heart transplantation (13).
The TAH is nearly identical to the is device descended from its predecessor the Jarvik-7 (Symbion, Inc., Salt Lake City) which was first implanted in 1982. In 1988, Dr. DeVries published in JAMA a detailed description of the surgical technique for implanting the Jarvik-7 (24). The pneumatically powered device weighed 480 g and was 10 cm × 10 cm × 15 cm in volume. Since that time, substantial surgical experience has accumulated at numerous centers across the country. More than 1,400 SynCardia TAH’s have been implanted into patients (15).
The CardioWest TAH consists of two polyurethane ventricles each with a stroke volume of 70 mL and occupies a volume of 400 cc within the chest (25). Given the size of the device, an anterior-posterior chest diameter of at least 10 cm is required by computed tomography (CT) from the anterior border of T10 vertebra to the posterior table of the sternum (24). Each chamber contains 2 mechanical single leaflet tilting disc valves [SynHall (formerly Medtronic Hall), 27 mm inflow, 25 mm outflow] to regulate direction of flow. The two ventricles are pneumatically actuated via drive lines that percutaneously attach to an external pump.
Prior to implantation, the Dacron aortic and pulmonary grafts are sealed using CoSeal Surgical Sealant (Baxter Healthcare Co., Freemont, CA) (25). The grafts and artificial ventricles are soaked in rifampin. A standard median sternotomy is performed. Two small incisions are made in the left upper abdomen and intramuscular tunnels are created through the left rectus muscle for the TAH drivelines. The drivelines are kept away from the midline to avoid injury and loss of pneumatic pressure during redo sternotomy. The patient is started on cardiopulmonary bypass. Mediastinal dissection and mobilization of the great vessels is minimized to maintain dissection planes for subsequent transplantation. The superior vena cava and inferior vena cava are cannulated via the right atrium. The aorta is cross clamped. The pulmonary artery and aorta are divided and separated at the level of the valvular commissures. The left and right ventricles are excised leaving a 1 cm rim of ventricular muscle around the mitral and tricuspid annulus. The mitral and tricuspid valve leaflets are excised. The coronary sinus is overseen. The atrial septum is inspected for a patent foramen ovale, which is closed if found. The TAH atrial quick connects are sutured to the respective valve annulus. Some institutions will reinforce this suture line with a strip of felt. The aortic and pulmonary artery graft quick connects are trimmed and sutured to the respective vessels. It is important that these are carefully cut to size to avoid both stretching and kinking. The pulmonary artery graft is longer than the aortic graft in order to reach over the aortic graft and connect to the artificial right ventricle (24).
At re-entry for transplantation, we have noted an intense inflammatory thickening of the pericardium that increases the difficulty of the mediastinal dissection (25). Polytetrafluoroethylene membrane (PTFE) (Preclude Pericardial Membrane, formerly called the Gore-Tex Surgical Membrane; W.L. Gore & Associates, Flagstaff, AZ) is used to fully reconstruct the pericardium. This maintains avascular tissue planes and dramatically simplifies re-entry for transplantation. Other centers have found wrapping the aortic anastomosis with a sterile tourniquet band to be helpful.
In contrast to the normal oblong cardiac silhouette, the TAH has an overall spherical configuration (25). While PTFE lining of the pericardium facilitates re-entry, contracture of the pericardium about the TAH can limit the space available for transplantation and require maneuvers to open the pericardium/pleura. A saline implant (Mentor smooth round, Mentor Worldwide LLC, Santa Barbara) placed at the former cardiac apex and inflated to 150-200 mL will adequately maintain this space and make such maneuvers unnecessary.
The drivelines are passed through the intramuscular tunnels in the left rectus with the Penrose drains (25). The TAH ventricles are attached to their respective atrial and arterial graft quick connects. An aortic root vent is placed and low pressure/low rate pumping (LV drive pressure 40 mmHg, rate 40 bpm, 40% systole, RV drive pressure 0 mmHg) of the left ventricle is started. Routine de-airing maneuvers are done and the aortic cross clamp is removed. De-airing is confirmed by transesophageal echocardiography (TEE). The patient is often readily weaned off cardiopulmonary bypass as TAH support is increased. Usual post bypass TAH parameters are left drive pressure 180-200 mmHg, right drive pressure 30-60 mmHg, HR 100-120 bpm, and 50% systole. Vacuum is usually not initiated until the chest is closed or sealed.
The decision on whether to close the chest immediately is made based on the bleeding risk (25). If coagulopathy is present, there is a low threshold for packing the mediastinum and delayed sternal closure. Compression of the TAH during chest closure can translate to compression of the left sided pulmonary veins, the inferior vena cava and the left bronchus. TEE is used at the end of the case to evaluate for adequate right and left atrial venous return. Should compression be identified it can generally be relieved by tethering the TAH anteriorly to the left costal margin.
Anticoagulation
Long term anticoagulation is routinely used in TAH patients to avoid thromboembolic complications. The initial safety and efficacy trial for the SynCardia TAH as bridge to transplantation reported strokes in 12% of patients and peripheral thrombotic events in 14% patients followed from enrollment to 30 days post-heart transplantation (11).
While the approach to anticoagulation in patients with TAH varies by institution, a multi-targeted antithrombotic approach including anticoagulants, antiplatelet, and rheologic agents are used.
This strategy was first introduced by Szefener at La Pitié Hospital (26). During 1,930 days of TAH support with either Jarvik-7 or CardioWest TAH-t, Szefener did not observe any strokes in patients who were treated with a combination of aspirin, dipyridamole, heparin, and pentoxifylline. Copeland and his colleagues published similar findings on thrombosis and bleeding outcomes on 99 patients with TAH who were treated with aspirin, unfractionated heparin or warfarin, dipyridamole and pentoxifylline (27). Strokes were observed at a rate of 2%. GI bleeding was observed in 4% of patients, intracranial bleeding at 2%, and late thoracic bleeding in 2%.
Anticoagulation begins post-operatively once homeostasis is achieved (28). Heparin is commonly used. As thrombocytopenia is common in critically ill patients requiring a TAH (particularly in patients with prior temporary MCS or CRRT), there is frequently a concern for heparin induced thrombocytopenia. We routinely use bivalirudin, a direct thrombin inhibitor, as our initial anticoagulation strategy. An aPTT goal of 50-70 is targeted. Once the patient is stable and tolerating oral intake well, they are bridged to warfarin anticoagulation with a target INR of 2-3. The antiplatelet medications aspirin 81 mg daily and dipyridamole 50 mg every 8 h are both started to maintain suppressed platelet function. Platelet function tests (e.g., light transmittance aggregometry) and the thromboelastogram (TEG) can be used to help titrate appropriate anticoagulation and platelet suppression.
The presence of four single leaflet mechanical valves in the TAH creates a constant amount of hemolysis (29). Similar to LVADs, the LDH, plasma free hemoglobin, and haptoglobin are monitored to assess the degree of hemolysis. Pentoxifylline is a rheologic agent that decreases blood viscosity, platelet adhesion, and increases red blood cell deformity and appears to improve the underlying hemolysis (28). Typical doses are 400 mg every 8 h.
The introduction of novel oral anticoagulants holds promise for improving the management of thromboembolic and bleeding complications in TAH patients.
Outcomes
The original safety and efficacy trial for the SynCardia TAH was responsible for first establishing the TAH as a relevant and effective intervention for bridging patients dying of biventricular heart failure to heart transplantation (14). As discussed earlier, patients were included who had class IV heart failure and hemodynamic insufficiency (hypotension, elevated CVP, on multiple vasoactive medications, IABP, or cardiopulmonary bypass). Patients were only chosen who were deemed to be poor LVAD candidates. Patients were effectively bridged to transplantation in an impressive 79% of patients.
To date, there have been no head-to-head randomized control trials comparing the efficacy of TAH with LVADs. One retrospective study published in 2001 compared the CardioWest TAH with Novacor and Thoratec LVADs; patients were effectively bridged to transplantation at a rate of 75%, 57%, 38%, respectively (19). Strokes were reported at a rate of 0.03 events per patient-month in CardioWest, 0.28 events per patient-month in Novacor LVAD, and 0.08 events per patient-month in Thoratec LVAD. In this study, it was observed that the patients who had poor outcomes in the LVAD groups were more likely to have concurrent right ventricular failure. The authors concluded that CardioWest be considered first-line in unstable patients who met device size parameters.
Another larger retrospective study of 383 patients published in the Journal of Heart and Lung Transplantation in 2012 selected patients from a multicenter French database and attempted to determine whether type of device, bi-ventricular assist devices or TAH, impacted rates of successful bridging to transplantation (20). This study found no statistically significant difference in rates of successful bridging to transplantation between patients treated with extracorporeal bi-ventricular assist devices, paracorporeal bi-ventricular assist devices, and CardioWest TAH . There was, however, a striking difference in the rates of stroke. Compared with the strokes reported in 61% and 57% in implantable and paracorporeal biventricular devices, respectively, strokes were reported in 16% of patients bridged with CardioWest TAH with a P value <0.001.
Our single center outcomes data was published in 2014. From April 2006 through July 2012 at Virginia Commonwealth University Medical Center, 66 patients were implanted with a TAH (25). Patients were supported for a median duration of 87.5 days. At the time of publication, 76% were successfully bridged to transplantation, 15% were discharged home on a portable Freedom Driver as part of a clinical trial, 11% remained on the device awaiting transplantation, and 14% died on the device.
Complications
As experience has grown with the TAHs, rates for the common complications have been established. The major complications of TAH implantation include strokes, infection, bleeding, thrombosis (discussed previously), renal failure, and chronic anemia. One center published outcomes data on 101 patients bridged with the TAH supported for an average of 87 days of support (30). Strokes were reported in 7.9% of patients, 63.4% developed an infection requiring treatment, and bleeding occurred in 42.6% of patients.
The lungs and the urinary tract system were the most common sites of infection. However, mediastinitis occurred in 3% of patients, two of whom died. There was one case of methicillin resistant staphylococcus aureus (MRSA) endocarditis complicated by multiple strokes and death.
Bleeding complications varied in severity. High rates of mediastinal bleeding requiring mediastinal exploration were observed in 24.7% of patients, 44% of whom died within one month. Approximately 4% developed gastrointestinal bleeding. Fifty-eight percent had no bleeding complications.
Renal dysfunction
Post-surgical oliguric renal failure is a frequent complication following TAH implantation. Severe renal dysfunction resulting in a rise in creatinine above 5 mg/dL or requiring dialysis is seen in up to 12% of patients post-operatively (31). One study found 15% of patients required renal replacement therapy who had no previous renal failure (32). Following the removal of the ventricles, B-type natriuretic peptide (BNP) levels drop precipitously (33,34). It has been postulated that interruption of this hormonal compensatory mechanism may precipitate renal failure (33,34). Patients who do develop oliguric renal failure have a prompt and robust increase in urine output following nesiritide infusion without worsening in hemodynamics (33,34). One small study looked at routinely administering low dose nesiritide (0.05 mcg/kg/min) to all patients undergoing TAH implantation at the time of ventriculectomy and demonstrated maintenance of urine output and GFR (34). While nesiritide administration appears to be beneficial in maintaining short term renal function and management of volume status, whether this results in a durable response compared with the natural history of renal recovery without the addition of nesiritide remains to be proven.
Longterm complications
While currently being investigated for use as destination therapy, the TAH is currently only approved for bridge to transplantation. As a result, data is lacking regarding long-term complications. As of 2011, 47 patients had been supported with a SynCardia TAH for greater than one year worldwide (35). The mean support time was 554 days. Device failure occurred in 10% of patients. Systemic infections were observed in 53% of patients, driveline infections in 27% of patients, thromboembolic events in 19% of patients, and hemorrhagic events in 14% of patients.
Chronic anemia
Severe anemia occurs following TAH implantation that is multifactorial in etiology. Levinson et al. demonstrated that hemolysis, similar to that seen following LVAD placement, occurs in patients following TAH implantation (36). The degree of anemia seen following TAH implantation is generally more severe than following LVAD placement. Mankad et al. published a study comparing anemia in 36 patients who underwent TAH implantation and 14 patients who underwent LVAD placement (29). Baseline hematocrits were similar between the two groups, and both groups experienced significant drops in hematocrit following device implantation. The anemia following TAH implantation, however, was statistically lower at 2, 4, 6 and 8 weeks following device implantation (P<0.001 for each). The researchers proposed multiple contributory mechanisms to the anemia. Evidence of severe hemolysis, like that which occurs in LVAD patients, was similarly found in TAH patients. Ninety-six percent of TAH patients had undetectable haptoglobin levels and elevated LDH (mean 1,128), and 40% of samples had detectable plasma free hemoglobin. They attributed this hemolysis to shear stress of multiple mechanical parts including the four mechanical valves and pneumatically powered diaphragms. Additionally, they proposed inflammation induced anemia to be playing a role as evidenced by elevated C-reactive protein which may be related to device materials. There was also evidence of inadequate hematopoiesis as demonstrated by a reduced reticulocyte production index. They hypothesized that this may also be mediated by inflammation. Interestingly, post-heart transplantation, the difference in hematocrit between LVAD and TAH disappeared, and by three months post heart transplantation hematocrit returned to baseline in both groups. Despite the severe anemia that occur post-TAH implantation, patients required a median of only 2.5 units of blood outside the post-operative period.
Future of the TAH
The TAH has an established role in the care of patients with biventricular heart failure as a bridge to transplantation. As discussed previously, the number of patients transplanted each year is outpaced by the number of patients waiting for heart transplant. On the frontier for TAH therapy is the expanded use to patients who are not candidates for heart transplantation. This type of application is referred to as destination therapy. On December 18, 2014, the FDA approved clinical study to evaluate safety and efficacy for the CardioWest TAH as destination therapy in 19 patients (37). Already in clinical use are portable “Freedom Drivers” which enable patients to be mobile and discharged from the hospital following device implantation (38). If approved for destination therapy, this would revolutionize the care of patients with end stage heart disease who are not transplant candidates.
Traditional chest wall size constraints of the TAH as discussed previously are being challenged by new technology. SynCardia has a designed a 50 cc version of its 70 cc predecessor which fits smaller patients with body surface areas down to 1.2 m2 (39). The FDA approved the smaller SynCardia TAH for use as a Humanitarium Use Device for adult patients at risk of imminent death due to cardiogenic shock and pediatric patients with congenital heart disease.
In addition to the well-established role in adult patients, there is an increased interest in use of the TAH in the pediatric patient population (23). This role may expand as smaller devices become available.
Other TAH devices
While this review has focused on the SynCardia TAH as it is the only commercially available TAH that is FDA approved, there are a few other notable devices that are currently under investigation. The AbioCor TAH is the first entirely implantable TAH with an internal battery that is charged transcutaneously (40). The results of the initial human trials were published in 2004. In total, seven patients underwent implantation, two of whom survived to hospital discharge. Two patients died early (one from intraoperative bleeding and another believed to be a reaction to aprotinin), and the other three patients died of a variety of complications including two from stroke which were felt to be related to thrombus formation on atrial struts. The overall 30-day survival was 71% and 43% at 60 days. This device is not currently in clinical use.
The BiVACOR TAH/BiVAD is a continuous flow magnetically powered designed to have the ability to either assist a failing heart or entirely assume the work of the heart. This is under development at the Texas Heart Institute (41). Cleveland Clinic has also developed a continuous flow TAH which has undergone animal testing in calves and human fit modeling (42,43).
Conclusions
The TAH is an important and effective intervention for patients who are dying of biventricular heart failure. Its role in destination therapy is on the horizon. As technology advances with the availability of the freedom driver, patients who have received a TAH are afforded more mobility and better quality of life.
Acknowledgements
None.
Footnote
Conflicts of Interest: Shah: Research Grants from Thoratec Corp; Tang: Principal Investigator for Syncardia Systems, HeartWare Corp and Sunshine Heart; Kasirajan: Principal Investigator for Thoratec Corp and Syncardia Systems.
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117-71. [PubMed]
- Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:996-1008. [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant 2014;33:555-64. [PubMed]
- Thanavaro KL, Tang DG, Cooke RH, et al. Characteristics and survival of patients with the total artificial heart implanted for indications other than biventricular failure. J Heart Lung Transplant 2013;32 suppl 4:S231.
- Cooley DA. In Memorium: Willem Johan Kolff 1911–2009. Tex Heart Inst J 2009;36:83-4.
- Barnard CN. The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J 1967;41:1271-4. [PubMed]
- Cooley DA, Liotta D, Hallman GL, et al. Orthotopic cardiac prosthesis for two-staged cardiac replacement. Am J Cardiol 1969;24:723-30. [PubMed]
- Curran WJ. Law-medicine notes. The first mechanical heart transplant: informed consent and experimentation. N Engl J Med 1974;291:1015-6. [PubMed]
- Pennock JL, Oyer PE, Reitz BA, et al. Cardiac transplantation in perspective for the future. Survival, complications, rehabilitation, and cost. J Thorac Cardiovasc Surg 1982;83:168-77. [PubMed]
- Cooley DA. The total artificial heart. Nat Med 2003;9:108-11. [PubMed]
- DeVries WC, Anderson JL, Joyce LD, et al. Clinical use of the total artificial heart. N Engl J Med 1984;310:273-8. [PubMed]
- Copeland JG, Levinson MM, Smith R, et al. The total artificial heart as a bridge to transplantation. A report of two cases. JAMA 1986;256:2991-5. [PubMed]
- Emery RW, Copeland JG. Heart transplantation in Arizona. J Heart Transplant 1985;4:203-5. [PubMed]
- Copeland JG, Smith RG, Arabia FA, et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med 2004;351:859-67. [PubMed]
- SynCardia Systems, Inc. Available online: http://www.syncardia.com
- Heartmate II by the numbers. Available online: http://heartmateii.com/heartmate-ii-by-numbers.aspx
- Shah KB, Smallfield MC, Tang DG, et al. Mechanical circulatory support devices in the ICU. Chest 2014;146:848-57. [PubMed]
- Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010;139:1316-24. [PubMed]
- Copeland JG 3rd, Smith RG, Arabia FA, et al. Comparison of the CardioWest total artificial heart, the novacor left ventricular assist system and the thoratec ventricular assist system in bridge to transplantation. Ann Thorac Surg 2001;71:S92-7; discussion S114-5.
- Kirsch M, Mazzucotelli JP, Roussel JC, et al. Survival after biventricular mechanical circulatory support: does the type of device matter? J Heart Lung Transplant 2012;31:501-8. [PubMed]
- Drakos SG, Janicki L, Horne BD, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol 2010;105:1030-5. [PubMed]
- Matthews JC, Koelling TM, Pagani FD, et al. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol. 2008;51:2163-72. [PubMed]
- Ryan TD, Jefferies JL, Zafar F, et al. The evolving role of the total artificial heart in the management of end-stage congenital heart disease and adolescents. ASAIO J 2015;61:8-14. [PubMed]
- DeVries WC. Surgical technique for implantation of the Jarvik-7-100 total artificial heart. JAMA 1988;259:875-80. [PubMed]
- Tang DG, Shah KB, Hess ML, et al. Implantation of the syncardia total artificial heart. J Vis Exp 2014;(89).
- Szefner J. Control and treatment of hemostasis in cardiovascular surgery. The experience of La Pitié Hospital with patients on total artificial heart. Int J Artif Organs 1995;18:633-48. [PubMed]
- Copeland J, Copeland H, Nolan P, et al. Results with an anticoagulation protocol in 99 SynCardia total artificial heart recipients. ASAIO J 2013;59:216-20. [PubMed]
- Ensor CR, Cahoon WD, Crouch MA, et al. Antithrombotic therapy for the CardioWest temporary total artificial heart. Tex Heart Inst J 2010;37:149-58. [PubMed]
- Mankad AK, Tang DG, Clark WB, et al. Persistent anemia after implantation of the total artificial heart. J Card Fail 2012;18:433-8. [PubMed]
- Copeland JG, Copeland H, Gustafson M, et al. Experience with more than 100 total artificial heart implants. J Thorac Cardiovasc Surg 2012;143:727-34. [PubMed]
- Copeland JG, Smith RG, Arabia FA, et al. Total artificial heart bridge to transplantation: a 9-year experience with 62 patients. J Heart Lung Transplant 2004;23:823-31. [PubMed]
- El-Banayosy A, Arusoglu L, Morshuis M, et al. CardioWest total artificial heart: Bad Oeynhausen experience. Ann Thorac Surg 2005;80:548-52. [PubMed]
- Delgado R 3rd, Wadia Y, Kar B, et al. Role of B-type natriuretic peptide and effect of nesiritide after total cardiac replacement with the AbioCor total artificial heart. J Heart Lung Transplant 2005;24:1166-70. [PubMed]
- Shah KB, Tang DG, Kasirajan V, et al. Impact of low-dose B-type natriuretic peptide infusion on urine output after total artificial heart implantation. J Heart Lung Transplant 2012;31:670-2. [PubMed]
- Torregrossa G, Morshuis M, Varghese R, et al. Results with SynCardia total artificial heart beyond 1 year. ASAIO J 2014;60:626-34. [PubMed]
- Levinson MM, Copeland JG, Smith RG, et al. Indexes of hemolysis in human recipients of the Jarvik-7 total artificial heart: a cooperative report of fifteen patients. J Heart Transplant 1986;5:236-48. [PubMed]
- FDA Approves the SynCardia Total Artificial Heart for Destination Therapy Study. Available online: http://www.syncardia.com/2015-multimedia-releases/fda-approves-the-syncardia-total-artificial-heart-for-destination-therapy-study/itemid-1737.html
- Demondion P, Fournel L, Niculescu M, et al. The challenge of home discharge with a total artificial heart: the La Pitie Salpetriere experience. Eur J Cardiothorac Surg 2013;44:843-8. [PubMed]
- Two Sizes Intended to Treat Most Patients. Available online: http://www.syncardia.com/medical-professionals/small-to-large-patients.html
- Dowling RD, Gray LA Jr, Etoch SW, et al. Initial experience with the AbioCor implantable replacement heart system. J Thorac Cardiovasc Surg 2004;127:131-41. [PubMed]
- Greatrex NA, Timms DL, Kurita N, et al. Axial magnetic bearing development for the BiVACOR rotary BiVAD/TAH. IEEE Trans Biomed Eng 2010;57:714-21. [PubMed]
- Fumoto H, Horvath DJ, Rao S, et al. In vivo acute performance of the Cleveland Clinic self-regulating, continuous-flow total artificial heart. J Heart Lung Transplant 2010;29:21-6. [PubMed]
- Karimov JH, Steffen RJ, Byram N, et al. Human Fitting Studies of Cleveland Clinic Continuous-Flow Total Artificial Heart. ASAIO J 2015;61:424-8. [PubMed]


