Standardized transbronchial needle aspiration procedure for intrathoracic lymph node staging of non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related motility and mortality worldwide, according to the latest data released by International Agency for Research on Cancer (IARC) (1). Early detection and precise staging are critical for clinical management of lung cancer. Metastasis in the intrathoracic lymph nodes (LNs), which is represented by the designator N in the TNM staging system, is usually a determining factor for therapeutic strategy for lung cancer. Computed tomography (CT) to determine LN staging of lung cancer relies on the size of malignant LNs and may only accurately detect malignant LNs that are larger than normal LNs. Toloza et al. found that 18% of the non-enlarged LNs that were detected by chest CT turned out to be malignant LNs after pathological examination (2), suggesting that LN size may not be a reliable parameter for evaluation of LN metastasis in patients with non-small cell lung cancer (NSCLC). In addition, mediastinoscopy, which shows a diagnostic sensitivity of 90-95%, is traditionally considered as the gold standard for mediastinal staging of NSCLC (3). However, nowadays, mediastinoscopy is no longer the first choice for lung cancer diagnosis and staging owing to the limitations in assessment and high invasiveness and risk (4,5).
Transbronchial needle aspiration (TBNA), which is considered as a remarkably invaluable and minimally invasive technique, has been widely used for the diagnosis and staging of mediastinal adenopathy and masses (6). LN staging may be misdiagnosed based on the assumption that the metastatic LNs usually present as enlarged LNs. Accumulating evidence support that endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) can improve the accuracy of diagnosis, but the procedure for EBUS-TBNA usually requires general anesthesia or conscious sedation, a second survey scope, and high expense (7-11). Most importantly, the misdiagnosis issue, which mentioned above, remains unsolved.
Here, we aim to address the misdiagnosis issue and investigate the effectiveness of a modified TBNA procedure to improve diagnostic sensitivity and accuracy in patients with NSCLC. We designed a standardized TBNA procedure and evaluated the effectiveness of the procedure. The standardized TBNA was performed in the order of N3-N2-N1 LN stations according to Wang’s LN map (12) and all LNs, including non-enlarged LNs on CT scan, were examined by TBNA.
Materials and methods
Patients
Participating patients were diagnosed with NSCLC between December 2014 and March 2015 by transbronchial biopsy, bronchial brushing, or CT-guided percutaneous needle aspiration with at least one LN station enlargement. Electronic bronchoscope (Olympus Corporation, CV-260SL, Tokyo, Japan) and Wang needle (MW-122) were used in this current study. The operations were performed under local anesthesia or concise sedation. This study was reviewed and approved by the Institutional Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. Informed consent was obtained from each patient.
Radiological assessment
High resolution CT (HRCT) was performed before bronchoscopy, and the results were reviewed by an experienced radiologist blindly. The cutoff diameter for enlarged LNs was 10 mm. A LN with a diameter >10 mm was considered as an enlarged LN. TNM staging of each patient was determined.
Standardized TBNA procedure
TBNA was carried out in the order of N3 to N1 LN station in each patient according to Wang’s LN map. Three passes were performed in each station. Specifically, the pass started from N3 stations (contralateral 2, 4, 10, and 11 stations), then N2 stations (ipsilateral 2, 4, 7, and 8 stations), eventually N1 stations (ipsilateral 10 and 11 stations). A small amount of the LN specimens was fixed in 95% ethanol and stained with H&E, and the remaining specimens were fixed in 10% formalin, embedded in paraffin, and stained with H&E. The H&E staining was reviewed by pathologists. TBNA was considered successful when the number of lymph cell was ≥40 per high power field or positive malignant lymph cells were detected. Positive diagnosis was defined as the detection of malignant lymph cells, and negative diagnosis was defined as the absence of malignant lymph cells.
Statistical analysis
Statistical analysis was performed using the statistical analysis software SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± standard deviation (SD). Metastatic rate and the success rate of biopsy are presented as rate ± standard error (SE). χ2 test was used to compare values of different groups. P<0.05 was considered statistically significantly different.
Results
TBNA results
A total of 53 patients, including 41 men and 12 women, participated in this study. The age of the patients was between 39 and 78 years, and the mean age was 62±10 years. Overall, 226 LNs from the 53 patients were sampled and 192 were biopsied successfully, representing a success rate of 84.96%. There were 116 LN metastasis, including 107 enlarged and 9 non-enlarged LNs. The metastatic rate for enlarged and non-enlarged LNs was 64.85% and 21.43%, respectively. The malignant non-enlarged LNs were at 4R, 7, 4L, 10L station (Table 1).
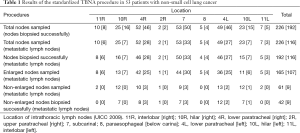
Full table
Comparison of metastatic rate in different regions
The LN stations, 2R, 4R, 4L, 7, and 8 were grouped into the mediastinal region, and 11R, 10R, 10L, 11L were considered in the hilar region. The proportion of metastatic LNs in non-enlarged LNs in the mediastinal region was significantly higher than that in the hilar region (24.24% vs. 3.57%, P=0.031). The success rate of TBNA in non-enlarged LNs was also dramatically higher in the mediastinal region than in the hilar region (84.84% vs. 50.00%, P=0.003) (Table 2).
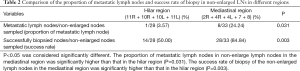
Full table
The effect of non-enlarged LNs on lung cancer staging
Compared with chest CT scan, TBNA detected 9 malignant non-enlarged LNs. Because of the detection of these malignant LNs by TBNA, the N staging was changed from N2 to N3 in 5 patients and from N1 to N2 in 3 patients (Table 3).
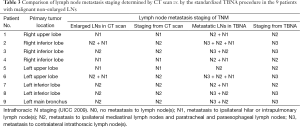
Full table
Skip LN metastasis
Of the 53 patients, 23 exhibited skip LN metastasis, including 1 solitary heterolateral hilar LN metastasis (4.35%), 8 solitary ipsilateral hilar LN metastasis (34.78%), 1 ipsilateral and heterolateral hilar without mediastinum nodal metastasis (N1 + N3, 4.35%), and 13 heterolateral hilar and mediastinum metastasis without affecting ipsilateral hilar LN (N3 + N2, 56.52%) (Table 4).
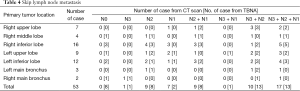
Full table
Complication
The 53 patients did not develop severe complications, such as hemorrhage, pneumothorax, pneumomediastinum, hematoma, or infection during the TBNA procedure.
Discussion
In this current study, we detected malignant metastasis in non-enlarged LNs by using a standardized TBNA procedure. Based on Wang’s LN map, we successfully biopsied 42 out of 61 non-enlarged LNs by the TBNA procedure and found that 9 out of the 42 LNs were metastatic LNs. This metastatic rate is consistent with the results from surgery (3-5,13). The slightly higher metastatic rate in this current study might be related with patient selection. Notably, 8 patients, who had non-enlarged LNs that were diagnosed as metastatic LNs by TBNA, eventually were re-staged to a more advanced LN metastasis stage, suggesting that detection of malignant non-enlarged LNs may be critical for lung cancer staging. Thus, we believe the standardized TBNA procedure in our current study may reduce misdiagnosis rate and improve the accuracy of LN metastasis staging in NSCLC.
Lymph drainage is the most common pathway for lung cancer metastasis. The hilar LNs are usually at the upstream of mediastinal LNs (14). However, mediastinal N2 LN metastasis may occur without the involvement of LNs in the hilar region, and these phenomena is called skip LN metastasis (15). In this current study, we detected skip LN metastasis by using the standardized TBNA procedure. Thus, our results indicate that N2 and N3 LNs should be examined for malignancy although N1 LNs present negative malignancy.
The proportion of metastatic LNs in non-enlarged LNs in the mediastinal region was approximately 7 times of that in the hilar region, and the success rate of TBNA in the mediastinal region was also dramatically higher than that in the hilar region. The low proportion of metastatic LNs in non-enlarged LNs in the hilar region may be associated with the poor success rate of TBNA, which was only 50%, in the hilar region. In addition, according to the principle of TNM staging in NSCLC, LN metastasis is defined based on the status of the contralateral LN but not the ipsilateral LNs. Thus, according to the operation duration of TBNA and patient tolerance, biopsy of non-enlarged LNs in the hilar region may be skipped without substantial effects on cancer staging.
High false negative rate is one of the disadvantages associated with TBNA for NSCLC staging. Previous studies showed that EBUS-TBNA presented a higher sensitivity (79-95%) with a lower false negative rate (1-37%) compared with conventional TBNA (13,16-20). The great variation (1-37%) in the false negative rate of the previous studies may be related with study design heterogeneity, LN size and station, TBNA method and passes, and the skill of the person who performed TBNA. False negative rate may be reduced by using the standardized TBNA procedure of this current study to decrease heterogeneity of TBNA.
Limitation
Positive results from TBNA usually do not require confirmation by surgery. Thus, the positive predictive value of the standardized TBNA procedure is 100%. However, negative results from TBNA need to be confirmed by surgery. Because the gold criteria by surgery were lacking in our study, we were unable to report the precise sensitivity and specificity of the standardized TBNA procedure.
Conclusions
In summary, we recommend the standardized TBNA procedure for LN biopsy. In this procedure, LNs should be biopsied in the order of N3 to N1 and both enlarged and non-enlarged LNs should be included to achieve accurate staging of lung cancer.
Acknowledgements
Funding: This work was supported by grants from the Project Grant from the Science & Technology Bureau of Wenzhou (Y20110094), a key platform project grant from the health department of Zhejiang (2015115320) and the research special fund for public welfare industry of health (201402024).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Stewart BW, Wild CP, editors. World Cancer Report 2014. Geneva: International Agency for Research on Cancer, 2014.
- Toloza EM, Harpole L, Detterbeck F, et al. Invasive staging of non-small cell lung cancer: a review of the current evidence. Chest 2003;123:157S-166S. [PubMed]
- Hoffmann H. Invasive staging of lung cancer by mediastinoscopy and video-assisted thoracoscopy. Lung Cancer 2001;34 Suppl 3:S3-5. [PubMed]
- Luke WP, Pearson FG, Todd TR, et al. Prospective evaluation of mediastinoscopy for assessment of carcinoma of the lung. J Thorac Cardiovasc Surg 1986;91:53-6. [PubMed]
- Coughlin M, Deslauriers J, Beaulieu M, et al. Role of mediastinoscopy in pretreatment staging of patients with primary lung cancer. Ann Thorac Surg 1985;40:556-60. [PubMed]
- Jiang J, Browning R, Lechtzin N, et al. TBNA with and without EBUS: a comparative efficacy study for the diagnosis and staging of lung cancer. J Thorac Dis 2014;6:416-20. [PubMed]
- Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest 2004;125:322-5. [PubMed]
- Navani N, Brown JM, Nankivell M, et al. Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: a multicenter study of 774 patients. Am J Respir Crit Care Med 2012;185:1316-22. [PubMed]
- Yarmus LB, Akulian JA, Gilbert C, et al. Comparison of moderate versus deep sedation for endobronchial ultrasound transbronchial needle aspiration. Ann Am Thorac Soc 2013;10:121-6. [PubMed]
- Pastis NJ, Simkovich S, Silvestri GA. Understanding the economic impact of introducing a new procedure: calculating downstream revenue of endobronchial ultrasound with transbronchial needle aspiration as a model. Chest 2012;141:506-12. [PubMed]
- Xia Y, Wang KP. Transbronchial needle aspiration: where are we now? J Thorac Dis 2013;5:678-82. [PubMed]
- Wang KP. Staging of bronchogenic carcinoma by bronchoscopy. Chest 1994;106:588-93. [PubMed]
- Herth FJ, Ernst A, Eberhardt R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically normal mediastinum. Eur Respir J 2006;28:910-4. [PubMed]
- Riquet M, Hidden G, Debesse B. Direct lymphatic drainage of lung segments to the mediastinal nodes. An anatomic study on 260 adults. J Thorac Cardiovasc Surg 1989;97:623-32. [PubMed]
- Prenzel KL, Baldus SE, Mönig SP, et al. Skip metastasis in nonsmall cell lung carcinoma: predictive markers and isolated tumor cells in N1 lymph nodes. Cancer 2004;100:1909-17. [PubMed]
- Herth FJ, Eberhardt R, Vilmann P, et al. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax 2006;61:795-8. [PubMed]
- Yasufuku K, Chiyo M, Koh E, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer 2005;50:347-54. [PubMed]
- Kanoh K, Miyazawa T, Kurimoto N, et al. Endobronchial ultrasonography guidance for transbronchial needle aspiration using a double-channel bronchoscope. Chest 2005;128:388-93. [PubMed]
- Plat G, Pierard P, Haller A, et al. Endobronchial ultrasound and positron emission tomography positive mediastinal lymph nodes. Eur Respir J 2006;27:276-81. [PubMed]
- Rintoul RC, Skwarski KM, Murchison JT, et al. Endobronchial and endoscopic ultrasound-guided real-time fine-needle aspiration for mediastinal staging. Eur Respir J 2005;25:416-21. [PubMed]


