
Prognostic factors and patients’ profile in treated stage I and II lung adenocarcinoma: a Hospital’s Cancer Registry-based analysis
Introduction
The incidence of lung cancer in both genders has risen over the last three decades and currently is the top-ranking cause of cancer-related deaths globally, accounting for 17% and 9% of all cancers in men and women, respectively (1-5). The latest GLOBOCAN [2020] study (6) reported that lung cancer is the most frequently diagnosed cancer with 11.7% of the total incident cancer cases in both genders, with an age-standardised incidence rate of 22.5 (31.5 in males and 14.6 in females) per 100,000 people. Lung cancer was also placed first and accounted alone for 1.8 million cancer-related deaths in 2020 (18.0% of the total), with an age-standardised mortality rate of 18.6 (25.9 in males, 11.2 in females) per 100,000 persons (5-15).
Despite curative-intent surgical resection, the estimated 5-year overall survival (OS) for patients with lung cancer stage I and II is 72% and 53%, respectively (16-18). The current standard of care for lung cancer patients at stages I and II is surgery. Despite the early diagnosis efforts, around 75% of patients are diagnosed with advanced disease on admission (stages III and IV) (19) and have a 1-year survival rate of only 15–19% compared with 81–85% for stage I (20).
Survival and prognostic factors of lung cancer patients have not been markedly known in many regions; even more unknown is the survival and profile of patients treated explicitly with stage I and II adenocarcinoma of the lung. It is already known that there are some variations in lung cancer survival between countries and among regions within countries (21).
The purpose of this study was to examine the long-term survival and possible predictors in all patients with stage I and II lung cancer adenocarcinoma with the Hospital’s Cancer Registry (HCR) responsible for the registry of cancer of the State of Sao Paulo, which is a geographical area with 40 million inhabitants.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-1071).
Methods
Patients, clinical stages (CS), and cancer registry
A Hospital-based retrospective cohort study included 1,278 adult patients diagnosed with adenocarcinoma of the lung (ICD-O 3rd ed. 8140/3), admitted with CS I and II (TNM 6th and 7th edition), between January 2000 to December 2015 and followed up until December 31, 2019.
All patients’ information was extracted from the HCR, coordinated by the Information and Epidemiology Directorate of the Sao Paulo Oncocentro Foundation (FOSP), responsible for the registry of cancer of the State of Sao Paulo (SISRHC) in 76 HCRs, and available on the FOSP website (https://www.fosp.saude.sp.gov.br/publicacoes/downloadarquivos). We included the patients with adenocarcinoma lung cancer stages I and II, older than 18 years, with confirmed histological lung cancer. It excluded the patients who had undergone previous treatment to any other neoplasm and patients with small cell lung cancer.
The variables analysed were sex, age at diagnosis, education, neighbourhood, CS at diagnosis, the time between diagnosis and treatment, 5-year periods in which patients were treated to assess possible survival improvement over time and treatment modality. This study was conducted based on the most common groups of the proposed treatment, including exclusive surgery, exclusive radiotherapy (Rxt) or adjuvant chemotherapy (QT) after surgery or adjuvant chemotherapy after radiotherapy. The patients were clustered into three groups: surgery, surgery plus other treatment and non-surgery group. The surgery group included patients undergoing surgery exclusively. Surgery plus other treatment groups included patients undergoing surgery followed by another treatment (adjuvant chemotherapy or radiotherapy in cases of compromised margins). The non-surgery group included patients treated without surgery (patients treated with radiotherapy and radiotherapy followed by adjuvant chemotherapy). Nodal status was confirmed by Thorax CT, PET-CT or invasive stage of the mediastinum with mediastinoscopy.
Specialised and partial-specialised hospitals were included. The Ministry of Health of Brazil defines the specialised hospitals as High Complexity Centers in Oncology (HCCO) and partial-specialised hospitals as Partial Hospital Complexity Centers in Oncology (PHCCO). HCCO are high-specialised hospitals with technical conditions, physical facilities, equipment, and human resources adequate to provide specialised high-complexity assistance for the definitive diagnosis and treatment of all types of cancer. HCCO must have radiotherapy equipment and assistance in their physical structure. PHCCO is defined as less-advanced hospitals with technical conditions, physical facilities, equipment, and human resources adequate to provide specialised assistance for the definitive diagnosis and treatment of the most prevalent cancers only and not all tumours. PHCCO must not have radiotherapy assistance installed in their physical structure.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee registered under protocol 49258615.4, and individual consent for this retrospective analysis was waived.
Statistical analysis
Descriptive analysis of the data was performed using absolute and relative frequencies and central tendency and dispersion measures. For the OS rates, patients diagnosed until December 31, 2014, were eligible. The difference between the date of vital status (death or alive) and the date of diagnosis (first appointment) were considered in calculating the OS. The date of death was confirmed by an active search [identification of vital status by the Users’ Registration of the Unified Health System (CADSUS)] and a passive search (linkage with the SEADE—Sao Paulo State Data Analysis System—Foundation and updating the hospitals with SISRHC).
According to predictor variables, the Kaplan-Meier limit product estimator test was applied to calculate the survival probability and compare the curves using the log-rank test. Cox univariate and multiple regression analyses were used to estimate the hazard ratio (HR) and its respective 95% confidence intervals (95% CI). For multiple modelling, variables with P<0.20 and clinical relevance were selected. The assumption of proportional hazards was based on Schoenfeld’s residual analysis. The data were analysed using the Statistical Package for the Social Sciences (SPSS) version 23 for Windows.
Results
Table 1 provides the primary patient characteristics, staging, and treatment. The study included 1,278 patients (53.5% male, 65.8% older than 60) diagnosed with lung adenocarcinoma in CS I (66.7%) and II. Most patients were treated in HCCO, 69% [882], while 31% [396] were treated in PHCCO. Most patients received surgery (surgery alone or surgery plus other treatment, 59.98%), and 55.8% of the patients started the treatment within two months (Table 1).
Table 1
| Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 684 (53.5) |
| Female | 594 (46.5) |
| Age group (years) | |
| <60 | 437 (34.2) |
| 60–69 | 443 (34.7) |
| >70 | 398 (31.1) |
| Schooling | |
| Illiterate | 54 (4.2) |
| Incomplete elementary school | 296 (23.2) |
| Complete elementary school | 289 (22.6) |
| High school | 131 (10.3) |
| Graduate | 98 (7.7) |
| Unknown | 410 (32.1) |
| Place of residence | |
| Metropolitan region | 803 (62.8) |
| Sao Paulo district | 409 (32.0) |
| Outsiders Sao Paulo State | 66 (5.2) |
| Specialized hospital | |
| HCCO | 882 (69.0) |
| PHCCO | 396 (31.0) |
| Clinical stage | |
| I | 853 (66.7) |
| II | 425 (33.3) |
| Prescribed treatment | |
| Surgery | 471 (36.9) |
| Surgery plus other treatment | 295 (23.1) |
| Non-surgery | 512 (40.1) |
| Time between diagnosing and the start of the treatment | |
| ≤2 months | 713 (55.8) |
| >2 months | 565 (44.2) |
| Status | |
| Alive with cancer | 110 (8.6) |
| Alive without information | 372 (29.1) |
| Death by cancer | 578 (45.2) |
| Death without information | 218 (17.1) |
| Total | 1,278 (100.0) |
FOSP, Oncocentro Foundation of Sao Paulo; HCCO, High Complexity Centers in Oncology; PHCCO, Partial Hospital Complexity Centers in Oncology.
The mean follow-up time for all patients was 45.2 months [standard deviation (SD) =41.3 months], with a median of 32.8 months, ranging from less than one to 233 months. The cumulative OS rate in 5 years was 39.3%. Male patients had a lower survival rate than females with a risk of death of HR =1.36 (95% CI: 1.17–1.58; P<0.001). In the age group, the patients 70 years or older showed a risk of death from HR =1.50 (95% CI: 1.25–1.79; P<0.001). Patients treated at PHCCO hospitals had lower 5-year OS rate than those treated in HCCO hospitals (30.5%; HR =1.50; 95% CI: 1.29–1.75; P<0.001) (Table 2).
Table 2
| Variables | Total/deaths | 5-year OS (%) months | (K-M) P value | Deaths HR (95% CI) | P value |
|---|---|---|---|---|---|
| Gender | <0.001 | <0.001 | |||
| Total | 1,142/655 | 39.3 | – | ||
| Female | 522/275 | 43.9 | 1 | ||
| Male | 620/380 | 35.4 | 1.36 (1.17–1.58) | ||
| Age group | <0.001 | ||||
| <60 | 401/208 | 44.8 | 1 | – | |
| 60–69 | 392/209 | 43.9 | 1.03 (0.86–1.24) | 0.751 | |
| ≥70 | 349/238 | 27.7 | 1.50 (1.25–1.79) | <0.001 | |
| Specialized hospitals | <0.001 | ||||
| HCCO | 783/417 | 43.2 | 1 | <0.001 | |
| PHCCO | 359/238 | 30.5 | 1.50 (1.29–1.75) | ||
| Place of residency | 0.012 | 0.023 | |||
| Sao Paulo State | 59/20 | 60.7 | 1 | ||
| Other | 1,083/635 | 38.3 | 1.60 (1.07–2.41) | ||
| Diagnose period | <0.001 | ||||
| 2000–2004 | 258/177 | 29.3 | 1 | – | |
| 2005–2009 | 352/215 | 37.1 | 0.85 (0.7–1.04) | 0.106 | |
| 2010–2014 | 532/263 | 46.2 | 0.65 (0.54–0.79) | <0.001 | |
| Clinical stage | <0.001 | <0.001 | |||
| I | 755/384 | 45.6 | 1 | ||
| II | 387/271 | 27.5 | 1.60 (1.37–1.86) | ||
| Time of diagnosing and treatment | 0.576 | 0.073 | |||
| ≤2 months | 662/378 | 40.1 | 1 | ||
| >2 months | 480/277 | 38 | 0.87 (0.75–1.01) | ||
| Treatment proposed | <0.001 | ||||
| Surgery | 412/149 | 60.8 | 1 | – | |
| Surgery and other | 342/196 | 39.7 | 1.72 (1.40–2.11) | <0.001 | |
| Non-surgery | 388/310 | 16.8 | 3.67 (3.04–4.44) | <0.001 |
OS, overall survival; FOSP, Oncocentro Foundation of Sao Paulo; HR, hazard ratio; CI, confidence interval; HCCO, High Complexity Centers in Oncology; PHCCO, Partial Hospital Complexity Centers in Oncology.
The time of diagnosing was grouped into 5 years and worked as an independent variable. Patients diagnosed between 2010 and 2014 had a protective factor against the risk of death concerning patients diagnosed between 2000 and 2004. In general, it was observed that OS was 45.6% in CS I and 27.5% in CS II (P<0.001), with increased risk of death (HR 1.60; 95% CI: 1.37–1.86; P<0.001) in patients with more advanced stage (Table 2).
Patients who underwent surgery plus other therapies or those who received non-surgical treatment had lower OS rates than patients who received only surgical treatment. The risk of death was HR =1.72 (95% CI: 1.40–2.11; P<0.001) and HR =3.67 (95% CI: 3.04–4.44; P<0.001) respectively, related to surgery plus other therapies and non-surgical patients. The complementary analysis showed a significant difference between the three treatment types (P<0.001—Kruskal-Wallis test). Median follow-up of surgical patients was 50.2 months (mean =56.4; SD =42.2), among those who received surgery plus other therapies, the median time was 39.2 months (mean =50.5; SD =41) and median time of 16.9 months (mean =28.5; SD =35.1) for non-surgical patients. Dunn’s post hoc test was applied. It was observed differences in the median follow-up time between the non-surgical and surgical patients and between the non-surgical and surgical plus other therapies patients (P<0.001) (Table 2).
Figure 1 shows the cumulative 5-year OS rate between age group, specialised status hospital, and treatment proposed.
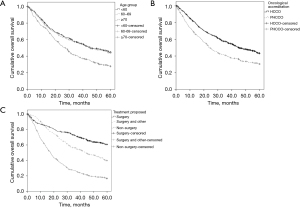
In the COX multiple regression analysis, the leading independent risk factors to death were gender, age, specialised hospitals, the proposed treatment, the time of diagnosing, and the CS at the diagnosis.
The female gender and the patients diagnosed between 2010 and 2014 have a lower risk of death. Patients aged 70 years or older were at increased risk of death from adjHR =1.44 (95% CI: 1.19–1.75; P<0.001), and those treated in non-high complexity centres had a risk of death adjHR =1.18 (95% CI: 1.00–1.40; P=0.047).
Likewise, patients treated with surgery plus other therapies or those who did not undergo surgery had a risk of death respectively, adjHR =1.48 (95% CI: 1.19–1.85; P=0.001) and adjHR =3.21 (95% CI: 2.60–3.97; P<0.001). Patients at CS II showed a risk of death of adjHR =1.34 (95% CI: 1.14–1.57; P<0.001) (Table 3).
Table 3
| Variables | Categories | adjHR (95% CI) | P value |
|---|---|---|---|
| Gender | Male | 1 | 0.009 |
| Female | 0.81 (0.69–0.95) | ||
| Age group | <60 | 1 | – |
| 60–69 | 1.04 (0.85–1.26) | 0.719 | |
| >70 | 1.44 (1.19–1.75) | <0.001 | |
| Specialised hospitals | HCCO | 1 | 0.047 |
| PHCCO | 1.18 (1.00–1.40) | ||
| Proposed treatment | Surgery | 1 | – |
| Surgery plus other | 1.48 (1.19–1.85) | 0.001 | |
| Non surgery | 3.21 (2.60–3.97) | <0.001 | |
| Time of diagnoses | 2000–2004 | 1 | – |
| 2005–2009 | 0.89 (0.73–1.09) | 0.27 | |
| 2010–2014 | 0.67 (0.55–0.82) | <0.001 | |
| Clinical stage | I | 1 | <0.001 |
| II | 1.34 (1.14–1.57) |
FOSP, Oncocentro Foundation of Sao Paulo; HR, hazard ratio; CI, confidence interval; HCCO, High Complexity Centers in Oncology; PHCCO, Partial Hospital Complexity Centers in Oncology.
Discussion
This study is the first that followed a large cohort of 1,278 patients diagnosed with adenocarcinoma of the lung in CS I and II focusing on the profile of patients treated in a region of about 40 million inhabitants. We observed that patients with lung cancer treated in HCCO have better median 5-year OS than patients with lung adenocarcinoma PHCCO. We also observed that the grouped 5-year time of diagnosing showed a 22% improvement of OS of patients diagnosed in 2010–2014 group (adjHR 0.67; 95% CI: 0.55–0.82; P<0.001) related to the 2005–2010 group (adjHR 0.89; 95% CI: 0.73–1.09) and 33% in comparison of 2000–2004 group (P<0.001).
Specialised and partial-specialised hospitals
This study discloses substantial differences in patients’ outcomes between HCCO and PHCCO in our region. These differences are influenced by the patient or tumour characteristics, but the hospital’s status seems to affect its treatment as well the expertise in cancer treatment and the availability of resources on site.
Specialised hospitals are often a matter of study, mainly examining the following guidelines among low-volume or high-volume institutions (22). High-volume oncologic hospitals were more likely to report high compliance in following the guidelines and be more accredited than low-volume hospitals (22). Antunez et al. (23) have found that patients treated in high-volume hospitals had better 5-year OS outcomes than those treated in low-volume hospitals (HR 0.99; 95% CI: 0.99–1.00; P<0.001). Our findings showed that 31% of patients were treated in PHCCO, leading to an increased risk of death (adjHR 1.53; 95% CI: 1.3–1.79; P<0.001) related to HCCO. Previous studies have found similar results, where many patients treated in high-complexity hospitals have better 5-year OS than non-high complexity hospitals. Mikami et al. (24) have compared Japanese institutions on women’s treatment and OS rates with cervical cancer. They have found that high-complexity institutions have significantly decreased mortality risk related to non-high complexity institutions (HR 0.843; 95% CI: 0.784–0.905; P<0.001). Similar findings were seen in women who received surgery alone (adjHR =0.552; 95% CI: 0.393–0.775) and received radiotherapy (adjHR =0.845; 95% CI: 0.766–0.931), showing improved survival in high-complexity institutions in Japan.
Differences in OS may be explained by differences between the staff’s expertise available in the large specialised high complexity and PHCCO as well as their more advanced and modern facilities in the first. New or improved treatment regimens usually are not equally implemented at the same time in all hospitals from the beginning, and these improvements may be initially available only in these high complexity hospitals. Otherwise, in large specialised hospitals, the high volume of patients can explain the difference in OS, considering the adoption of innovative treatment and advanced facilities and infrastructures (25).
In our study, HCCO shows better OS. The differences in treatments and survival rates between hospital types can be a combination of skills and experience of the medical team, as well the multidisciplinary team in staging (25). Despite this, some studies argue that the hospital infrastructures and methods of delivering health care in a multidisciplinary staff may be more important than the expertise of the individual surgeon, suggesting that cancer care outcomes were more strongly associated with the hospital volume than with the surgeon volume (22).
Therapies and stage
The oncological treatment is exceptionally patient- and tumour-dependent, and this heterogeneity directly influences OS (12,26,27).
Our study shows the most frequent treatments for these patients regarding the therapies’ combined modalities. The patient treated with surgery alone (36.9%) has the highest 5-year OS time (60.8 months) in comparison to those who received surgery plus other therapies (39.7 months) and non-surgical treatment (16.8 months). Patients who underwent surgery and other therapies have an almost 50% increased risk of death than those who only underwent surgery (HR 1.48; 95% CI: 1.19–1.85; P=0.001). Patients who do not have surgery conditions have more than three times an increased risk of death than those who have only undergone surgery (HR 3.21; 95% CI: 2.60–3.97). It is important to emphasize that it was not possible to know whether patients undergoing the best supportive care were included in the non-surgical group. If this happened, the HR risk measure is probably overestimated. We observed that OS was 27.5% in CS II and 45.6% in CS I. Two findings may explain these survival results. First, about 44% of our patients have waited more than two months to start the treatment after the staging. This delay in receiving the fitted treatment may have influenced the patients’ OS in some ways. The second finding is that 40.06% of the patients did not receive surgical treatment (Table 1), probably due to the lack of clinical conditions or refusal of surgical treatment. The fact that follow-up was statistically lower in the non-surgical group, according to Dunn’s post hoc test described in the results section, suggests that the clinical conditions of these patients may also have favoured early death. However, the shorter follow-up may also be a consequence of the treatment for lung cancer, not including surgery.
About prolonged waiting time between the diagnosis and the start of the treatment may also lead to lower survival and high mortality rates (28). The time between the first appointment with the general practitioner and the specialised care is also critical and crucial to define the patient’s survival (7,29-31). The clinical-stage at admission reflects the patient’s understanding of signals and symptoms, how and where they will find help after the first contact with outpatient care, the screening process, and the referral process.
Official guidelines concerning the diagnosis and treatment of lung cancer recommend a maximum delay of 7–14 days between the first appointment and the specialist and 28 days between the diagnosis and the surgery (32,33). Despite this, in a literature review, Olsson et al. showed that median times to diagnose and treat lung cancer ranged from 8 to 60 and 30 to 84 days, respectively, often exceeded published recommendations (34). These delays are likely to affect treatment outcomes and, therefore, the disease’s prognosis (34-37). In a previous study of our group, we have seen a similar scenario that one of the multivariable specific risk factors associated with lung cancer CS II survival was delay treatment (HR 3.08; 95% CI: 1.05–9.0; P=0.04) (38,39).
The time of the diagnose strongly interferes with 5-year OS. In our study, the 5-year OS increased 16.9% and 7.8% in the 2010–2014 and 2005–2009 group compared to the 2000–2004 group of treated patients. Patients diagnosed more recently have a lower risk of death than those diagnosed in later years. The 5-year group [2014–2010] has adjusted hazard risk to death compared to the 2000–2004 group, with an adjusted HR 0.67 (95% CI: 0.55–0.82).
This particularity may reflect the substantial recent advances in the overall treatment of cancer patients as new diagnostic and staging tools, surgical procedures, as well as the follow-up and improved access to public health care. It should be noted that one of the reasons for improved survival over the 5-year periods was the new versions of the TNM staging, which better stratified the survival of patients with lung cancer. As a result, all the treatment decisions or multiple therapies have changed in the last decade, guided by the general guidelines that help to decide the best step to be made at each stage of the disease. Nevertheless, the variation in the 5-year OS rates can mean that some improvements can be made in treating adenocarcinoma cancer patients in our region.
Our study’s findings are similar to others that found decreased deaths from NSCLC faster than the decrease in NSCLC incidence in recent years. Howlader et al. (40) have found an annual decrease of 3.2% in deaths from lung cancer between 2006 to 2013 and 6.3% from 2013 to 2016, higher than the 1.9% and 3.1% decrease in the lung cancer incidence. To reinforce these findings, the 2-year survival for patients with NSCLC improved from 26% for patients diagnosed in 2001 to 35% for those diagnosed in 2014. Jones et al. (41) shows that the 1-year survival of lung cancer patients in England has improved from 24.5% in 1995–1999 to 36.7% in 2018, resulted by new improvements in lung cancer care. Walters et al. in a recent study in Europe have observed that for some cancers, including lung cancer, the 1-year survival improved 1% annually between 2005–2009 and 2% during 2010–2012 in comparison to 1995–1999 (42).
Limitation
Our study has several limitations. First, internal differences in therapeutic modalities between hospitals were not available, as well as the patients’ characteristics in each type of hospital. Second, the frequency of PET-CT and invasive mediastinal staging was not evaluated. Third, we did not have complete data on whether all operations were complete and performed according to the IALSC guidelines. Consequently, we did not analyse the number of dissected mediastinal lymph nodes or the surgical margins for each patient, impacting survival. Finally, the study relies on data already collected, and therefore any systematic errors occurring during the chart abstraction process cannot be captured.
Conclusions
In conclusion, despite the differences between the complexity of hospitals, the 5-year OS has significantly improved as long as the 5-year group analysed. Also, the 5-year OS of the patients treated in high complexity hospitals is higher than those treated in PHCCO. These findings probably reflect an improvement in clinical and surgical oncology treatment over the 5 years analysed, as well as the establishment of an adequate line of care in high complexity hospitals.
Acknowledgments
The authors thank the Epidemiology Directorate of Oncocentro Foundation of Sao Paulo (FOSP). In addition, all the administrative staff was responsible for the data acquisition and cancer registry of the State of Sao Paulo (SISRHC).
Funding: This article is partially sponsored by a grant from the Onconcentro Foundation of Sao Paulo (FOSP). This sponsor did not influence the writing in any way.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-1071
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-1071). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee registered under protocol 49258615.4, and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Cheng TY, Cramb SM, Baade PD, et al. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. J Thorac Oncol 2016;11:1653-71. [Crossref] [PubMed]
- Lin HT, Liu FC, Wu CY, et al. Epidemiology and Survival Outcomes of Lung Cancer: A Population-Based Study. Biomed Res Int 2019;2019:8148156 [Crossref] [PubMed]
- Beck N, Hoeijmakers F, van der Willik EM, et al. National Comparison of Hospital Performances in Lung Cancer Surgery: The Role of Case Mix Adjustment. Ann Thorac Surg 2018;106:412-20. [Crossref] [PubMed]
- Romaszko AM, Doboszyńska A. Multiple primary lung cancer: A literature review. Adv Clin Exp Med 2018;27:725-30. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Wouters MW, Siesling S, Jansen-Landheer ML, et al. Variation in treatment and outcome in patients with non-small cell lung cancer by region, hospital type and volume in the Netherlands. Eur J Surg Oncol 2010;36:S83-92. [Crossref] [PubMed]
- Ricardo-Ramírez C, Álvarez-Gómez M, Ocampo-Saldarriaga MV, et al. Prevalencia de tamizaje positivo para depresión y ansiedad en gestantes de alto riesgo obstétrico en una clínica de Medellín, entre enero y agosto de 2013. Factores de riesgo asociados. Rev Colomb Obstet Ginecol 2015;66:94. [Crossref]
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018;103:356-87. [Crossref] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204-22. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Hutchinson BD, Shroff GS, Truong MT, et al. Spectrum of Lung Adenocarcinoma. Semin Ultrasound CT MR 2019;40:255-64. [Crossref] [PubMed]
- van der Linden N, Bongers ML, Coupé VMH, et al. Treatment Patterns and Differences in Survival of Non-Small Cell Lung Cancer Patients Between Academic and Non-Academic Hospitals in the Netherlands. Clin Lung Cancer 2017;18:e341-7. [Crossref] [PubMed]
- Hanna N, Johnson D, Temin S, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484-515. [Crossref] [PubMed]
- Jacobsen MM, Silverstein SC, Quinn M, et al. Timeliness of access to lung cancer diagnosis and treatment: A scoping literature review. Lung Cancer 2017;112:156-64. [Crossref] [PubMed]
- Eguchi T, Kadota K, Chaft J, et al. Cell cycle progression score is a marker for five-year lung cancer-specific mortality risk in patients with resected stage I lung adenocarcinoma. Oncotarget 2016;7:35241-56. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Kay FU, Kandathil A, Batra K, et al. Revisions to the Tumor, Node, Metastasis staging of lung cancer (8th edition): Rationale, radiologic findings and clinical implications. World J Radiol 2017;9:269-79.
- Blandin Knight S, Crosbie PA, Balata H, et al. Progress and prospects of early detection in lung cancer. Open Biol 2017;7:170070 [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Skaug K, Eide GE, Gulsvik A. Predictors of long-term survival of lung cancer patients in a Norwegian community. Clin Respir J 2011;5:50-8. [Crossref] [PubMed]
- Lee L, Dietz DW, Fleming FJ, et al. Accreditation Readiness in US Multidisciplinary Rectal Cancer Care: A Survey of OSTRICH Member Institutions. JAMA Surg 2018;153:388-90. [Crossref] [PubMed]
- Antunez AG, Kanters AE, Regenbogen SE. Evaluation of Access to Hospitals Most Ready to Achieve National Accreditation for Rectal Cancer Treatment. JAMA Surg 2019;154:516-23. [Crossref] [PubMed]
- Mikami M, Shida M, Shibata T, et al. Impact of institutional accreditation by the Japan Society of Gynecologic Oncology on the treatment and survival of women with cervical cancer. J Gynecol Oncol 2018;29:e23 [Crossref] [PubMed]
- Cardarella S, Ortiz TM, Joshi VA, et al. The introduction of systematic genomic testing for patients with non-small-cell lung cancer. J Thorac Oncol 2012;7:1767-74. [Crossref] [PubMed]
- Uhlig J, Case MD, Blasberg JD, et al. Comparison of Survival Rates After a Combination of Local Treatment and Systemic Therapy vs Systemic Therapy Alone for Treatment of Stage IV Non-Small Cell Lung Cancer. JAMA Netw Open 2019;2:e199702 [Crossref] [PubMed]
- de Castro J, Tagliaferri P, de Lima VCC, et al. Systemic therapy treatment patterns in patients with advanced non-small cell lung cancer (NSCLC): PIvOTAL study. Eur J Cancer Care (Engl) 2017;26:e12734 [Crossref]
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 2015;112:S92-107. [Crossref] [PubMed]
- Pérez G, Porta M, Borrell C, et al. Interval from diagnosis to treatment onset for six major cancers in Catalonia, Spain. Cancer Detect Prev 2008;32:267-75. [Crossref] [PubMed]
- Olesen F, Hansen RP, Vedsted P. Delay in diagnosis: the experience in Denmark. Br J Cancer 2009;101:S5-8. [Crossref] [PubMed]
- van de Ven M, Retèl VP, Koffijberg H, et al. Variation in the time to treatment for stage III and IV non-small cell lung cancer patients for hospitals in the Netherlands. Lung Cancer 2019;134:34-41. [Crossref] [PubMed]
- BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax 1998;53:S1-8. [Crossref] [PubMed]
- Alberts WM, Bepler G, Hazelton T, et al. Lung cancer. Practice organization. Chest 2003;123:332S-7S. [Crossref] [PubMed]
- Olsson JK, Schultz EM, Gould MK. Timeliness of care in patients with lung cancer: a systematic review. Thorax 2009;64:749-56. [Crossref] [PubMed]
- Giroux Leprieur E, Labrune S, Giraud V, et al. Delay between the initial symptoms, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin Lung Cancer 2012;13:363-8. [Crossref] [PubMed]
- Salomaa ER, Sällinen S, Hiekkanen H, et al. Delays in the diagnosis and treatment of lung cancer. Chest 2005;128:2282-8. [Crossref] [PubMed]
- O'Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol) 2000;12:141-4. [Crossref] [PubMed]
- Abrao FC, Abreu IRLB, Rocha RO, et al. Impact of the delay to start treatment in patients with lung cancer treated in a densely populated area of Brazil. Clinics (Sao Paulo) 2017;72:675-80. [Crossref] [PubMed]
- Abrao FC, de Abreu IRLB, Rocha RO, et al. Interaction between treatment delivery delay and stage on the mortality from non-small cell lung cancer. J Thorac Dis 2018;10:2813-9. [Crossref] [PubMed]
- Howlader N, Forjaz G, Mooradian MJ, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med 2020;383:640-9. [Crossref] [PubMed]
- Jones GS, Baldwin DR. Recent advances in the management of lung cancer. Clin Med (Lond) 2018;18:s41-6. [Crossref] [PubMed]
- Walters S, Benitez-Majano S, Muller P, et al. Is England closing the international gap in cancer survival? Br J Cancer 2015;113:848-60. [Crossref] [PubMed]


