
The role of long-acting muscarinic antagonist/long-acting β agonist fixed-dose combination treatment for chronic obstructive pulmonary disease in China: a narrative review
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease involving systemic inflammation characterized by persistent airflow limitation caused by increased small airway resistance and decreased lung compliance (1,2). COPD has become the third leading cause of death in China, and it imposes a substantial disease burden due to the associated economic burden and reduction in quality of life (QoL) (3,4). The main symptoms of COPD are dyspnea, cough, and sputum production with lung hyperinflation, which substantially impact patients’ lives (5). Systemic inflammation in COPD is associated with multiple comorbidities, which may lead to poor clinical outcomes (for instance, more symptomatic and worse health status) and higher mortality (6). In Chinese patients, COPD results in direct medical costs ranging from 72 to 3,565 USD per capita per year, which is equivalent to 33.33% to 118.09% of the local average annual income (4).
Approximately 60% of COPD patients in China are smokers, and the proportion in male patients is greater than 80% (7). Smoking is an important cause of respiratory symptoms. A total of 67.3% of outpatients with COPD in China had an ability of the forced expiratory volume in one second calculated as a percentage of the forced vital capacity (FEV1%) lower than 50% (8), and their symptoms were more severe than those of patients in Europe or America (9). Only one-third of the patients with a potential diagnosis of Global Initiative for Obstructive Lung Disease (GOLD) stage I COPD actually have a respiratory disease diagnosis, and more than one-third of patients with a potential diagnosis of COPD are asymptomatic (10). Although substantial attention has been given to COPD management in recent years in China, this disease remains underdiagnosed, and the pharmacotherapy options available to patients are still inadequate (11).
As COPD is a chronic disease, long-term maintenance treatment is essential. The main aims of the pharmacological management of COPD are relieving patients’ symptoms and reducing the risk of exacerbations, hence increasing patients’ ability to engage in daily activities and supporting their QoL (12). Inhaled bronchodilators are the mainstay of therapy at each stage of the disease. However, more than half of Chinese COPD patients live in rural areas, and most of them are undertreated; the use of inhalation therapy is very rare (13). A study based on COPD disease surveillance data from 2014
We present the following article in accordance with the Narrative Review reporting checklist (available at available at https://dx.doi.org/10.21037/jtd-21-961).
Methods
Search strategy and selection criteria
In this review, clinical trials on LAMA/LABA dual bronchodilator treatment in COPD included were retrieved in ClinicalTrials.gov, and main guidelines regarding COPD published before February 2021 in either English or Chinese were identified by searches in PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Wanfang Database (http://www.wanfangdata.com.cn). Wanfang database, which is affiliated to the Chinese Ministry of Science & Technology, offers access to variety of databases, including medicine databases.
Pharmacological properties of LAMA/LABA combinations
Airway resistance is jointly controlled by the sympathetic nervous system and the parasympathetic nervous system. The mechanisms that cause airway relaxation and contraction interact with and enhance each other; although they can act independently, they often act in combination (17,18). The bronchodilation effect of LAMAs is produced by the precise competitive and reversible inhibition of endogenous acetylcholine, thereby inhibiting the activation of muscarinic acetylcholine receptor M3 (M3 receptors) (19). The bronchodilation effect of LABAs is produced by the activation of β2-adrenergic receptors (β2-AR), which are widely distributed throughout the respiratory system. LABAs relax airway smooth muscles, inhibit the release of inflammatory mediators, and reduce the release of acetylcholine, and effectively relaxing airways at all levels (20).
Rather than just increasing the dose of bronchodilators, dual bronchodilator treatment with LAMAs and LABAs has a synergistic effect (21,22). The definition of synergy is that the therapeutic effect of the drug combination is greater than the sum of the therapeutic effects of the individual monotherapies. Owing to its synergistic effects, dual bronchodilator treatment can achieve a greater degree of bronchodilation with lower doses of the individual components, thereby minimizing the side effects. A synergistic effect also exists at the molecular level. LABAs continuously and rapidly increase the intracellular cyclic adenosine monophosphate (cAMP) concentration and therefore effectively functionally antagonize airway smooth muscle contraction, which also leads to muscarinic receptor postjunctional competitive antagonism (23). Activation of the Gq protein coupled to M3 receptors can be inhibited by LABAs through the transcriptional stimulation of G protein signal transduction 2 regulator expression, thereby specifically inhibiting Gq protein activation (24). LAMAs can prevent β2-AR desensitization, thereby enhancing bronchodilation. LAMAs block the M3 receptor and hence inhibit the coupling of the receptor to the signal transduction pathway, thereby sequentially activating Gq and Phospholipase C (PLC), which leads to diglyceride (DAG) synthesis. DAG is the intracellular second messenger that activates protein kinase C (PKC), which leads to β2-AR and Gs protein phosphorylation and hence the disassociation and coupling of two main components in the signaling pathway underlying bronchodilation induced by β2-adrenergic agonists (24). Additionally, LABAs inhibit the prejunctional release of acetylcholine (Ach), which enhances the bronchodilation induced by LAMAs (23). Therefore, LAMA/LABA combination therapies can utilize the adrenergic and cholinergic pathways in airway smooth muscles to maximize bronchodilation (24). The coadministration of LAMAs and LABAs synergistically induces increases in human isolated medium-sized airway smooth muscle relaxation and small airway lumen area (25). Further forced expiratory volume in one second (FEV1) improvement is the result of the synergistic action of dual bronchodilator therapy compared with the effects of monotherapy on medium-sized airways, while the synergistic effect on small airways is reflected in the benefit observed lung hyperinflation, which is observed as a reduction in symptoms, increased exercise capacity, and reduced risk of exacerbation (21). LAMA/LABA FDCs are more convenient than free combinations of LAMAs and LABAs, thereby enhancing adherence. Besides, the dose of each medication in combination can be optimized during the development (26). In recent years, a number of LAMA/LABA FDCs have been developed. Table 1 shows the information regarding the existing LAMA/LABA FDCs on the market. Among these medicines, glycopyrronium/indacaterol (GLY/IND), umeclidinium/vilanterol (UMEC/VI), tiotropium/olodaterol (TIO/OLO), and glycopyrronium/formoterol (GFF) are currently available on the market on the Chinese mainland.
Table 1
| Variable | Glycopyrronium/indacaterol | Umeclidinium/vilanterol | Aclidinium/formoterol | Tiotropium/olodaterol | Glycopyrronium/formoterol | Tiotropium/formoterol |
|---|---|---|---|---|---|---|
| Abbreviation | GLY/IND | UMEC/VI | ACL/FF | TIO/OLO | GFF | TIO/FF |
| Delivered dose (μg) | 50/110 | 62.5/25 | 400/12 | 2.5/2.5 | 7.2/5 | 18/12 |
| Frequency | One inhalation once daily | One inhalation once daily | One inhalation twice daily | Two puffs once daily | Two inhalations twice daily | One puff once daily |
| Approved in China | √ | √ | – | √ | √ | – |
GLY/IND, glycopyrronium/indacaterol; UMEC/VI, umeclidinium/vilanterol; ACL/FF, aclidinium/formoterol; TIO/OLO, tiotropium/olodaterol; GFF, glycopyrrolate/formoterol fumarate; TIO/FF, tiotropium/formoterol.
LAMA/LABA combination therapy in the world
Globally comprehensive clinical evidence regarding LAMA/LABA FDCs
At present, there are various ongoing clinical trials of the LAMA/LABA FDCs mentioned above, and some of the results from Phase III and Phase IV studies have been released, providing us with a substantial amount of clinical evidence regarding the therapeutic effect and safety of these LAMA/LABA FDCs. Table 2 show the current main clinical trials of these LAMA/LABA FDCs. Rodrigo et al. (27) conducted a systematic review on the efficacy and safety of LAMA/LABA FDCs compared with LAMA monotherapy or LABA/ICS combinations; randomized, parallel-group, controlled trials with durations longer than 4 weeks were included. Compared with bronchodilator monotherapies, all LAMA/LABA FDCs treatments improved lung function, the transition dyspnea index (TDI), the St. George’s Respiratory Questionnaire (SGRQ) score and the exacerbation rate (27,28). LAMA/LABA FDCs also improved patients’ exercise capacity; the improvements in endurance and inspiratory capacity of the LAMA/LABA FDCs group compared with the monotherapy group met the putative clinically meaningful differences (29,30). In studies comparing LAMA/LABA FDCs versus LABA/ICS, LAMA/LABA FDCs seemed to result in greater improvements in lung function parameters (31). In terms of exacerbations, GLY/IND treatment significantly reduced the annual moderate and/or severe exacerbation rate (32). Regarding safety outcomes, LAMA/LABA FDCs were not significantly different from monotherapies (33). The adverse event rates and porportion of withdrawal of treatment due to adverse events in patients receiving LAMA/LABA treatment were significantly lower than those in patients receiving LABA/ICS treatment (27); LAMA/LABA FDCs are associated with fewer severe cases of pneumonia (34). The CLAIM study showed that GLY/IND significantly reduced hyperinflation, and this improvement could lead to an improvement in cardiac function (35).
Table 2
| LAMA/LABA FDCs | Clinical trials |
|---|---|
| GLY/IND | NCT01120691 (SPARK); NCT01202188 (SHINE); NCT01727141 (FLIGHT1); NCT01712516 (FLIGHT2); NCT01529632 (BEACON); NCT01709903 (LANTERN); NCT01315249 (ILLUMINATE); NCT01782326 (FLAME) |
| UMEC/VI | NCT01313637; NCT01316900; NCT01316913; NCT01313650; NCT01716520; NCT01491802 |
| GFF | NCT01587079; NCT01854645 (PINNACLE 1); NCT01854658 (PINNACLE 2) |
| TIO/OLO | NCT01431274 (TONADO 1); NCT01431287 (TONADO 2); NCT01525615 (TORRACTO); NCT01533922 (MORACTOTM 1); NCT01533935 (MORACTOTM 2); NCT01559116; NCT01536262 |
| ACL/FF | NCT01572792 (AUGMENT); NCT01462942 (ACLIFORM); NCT01437540; NCT01049360 (LAC-MD-27) |
| TIO/FF | NCT02988869 |
LAMA, long-acting muscarinic antagonist; LABA, long-acting β agonist; FDC, fixed-dose combination; GLY/IND, glycopyrronium/indacaterol; UMEC/VI, umeclidinium/vilanterol; GFF, glycopyrrolate/formoterol fumarate; TIO/OLO, tiotropium/olodaterol; ACL/FF, aclidinium/formoterol; TIO/FF, tiotropium/formoterol.
Global guidelines and consensus
Numerous guidelines have been published worldwide that are continuously updated to provide a standardized clinical reference based on the latest evidence for the diagnosis, treatment and prevention of COPD. The summary of changes in recommendations regarding dual bronchodilator therapy in the guidelines mentioned in this section is shown in Table 3. One of the most influential is the GOLD report, which was first published in 2001 and has been continuously updated thereafter (36). From GOLD document 2011, COPD patients are divided into four groups (Groups A to D) based on their dyspnea symptoms and acute exacerbations (36-38). LAMA/LABA is first recommended for Group B patients with severe breathlessness as second choice, and an alternative for Group C and D patients in GOLD 2013 (12). In GOLD 2017, LAMA/LABA is still recommended to Group B, C and D patients, with more detailed restrictions on which patients need LAMA/LABA. In Group B, patients with severe breathlessness may consider LAMA/LABA as initial therapy, and LAMA/LABA is also recommended for patients with persistent breathlessness on monotherapy. Group C with persistent exacerbations and Group D patients may choose LAMA/LABA (38). From the GOLD document 2019, the recommendations for medical treatment are divided into initial treatment and follow-up. GOLD groups of patients are not considered in the medical treatment recommendation during follow-up (37). Until the latest version of the GOLD report (update 2021), for initial treatment, LAMA/LABA is recommended to Group D patients. In follow-up, patients who still have dyspnea or/and exacerbation on bronchiolar monotherapy should use dual therapy (36).
Table 3
| Guidelines | Year | Recommendations |
|---|---|---|
| GOLD | 2021* | Initial treatment: LAMA/LABA combination therapy may be chosen for Group D patients. Follow-up: patients who still have dyspnea or/and exacerbation on bronchiolar monotherapy should use dual therapy |
| 2017 | Group B: for patients with persistent breathlessness on monotherapy, the use of two bronchodilators is recommended. For patients with severe breathlessness, initial therapy with two bronchodilators may be considered. Group C: patients with persistent exacerbations may benefit from LAMA/LABA combination therapy. Group D: Patients should receive LAMA/LABA combination therapy | |
| 2013 | Group B: for patients with severe breathlessness, the second choice is LAMA/LABA combination therapy. Group C: dual bronchodilator therapy is an alternative choice. Group D: dual bronchodilator therapy is an alternative choice | |
| GesEPOC | 2017 | Low-risk: patients who still have symptoms or clear exercise limitations on bronchodilator monotherapy should use dual bronchodilator therapy. High-risk: patients with the nonexacerbated phenotype, exacerbated with emphysema phenotype, and exacerbated with chronic bronchitis phenotype should use LAMA/LABA combination therapy as an initial treatment |
| 2014 | Nonexacerbated phenotype: LAMA/LABA can be used in patients with severity levels of II and III. Exacerbated with emphysema phenotype and exacerbated with chronic bronchitis phenotype: LAMA/LABA is can be selected for patients with severity level II | |
| NICE | 2018 | LAMA/LABA is recommended for people who: (I) have spirometrically confirmed COPD and; (II) have no asthmatic features/features suggesting steroid responsiveness and; (III) remain breathless or have exacerbations despite having used or been offered treatment for tobacco dependence if they smoke, optimized nonpharmacological management, relevant vaccinations, and a short-acting bronchodilator |
| ATS | 2020 | LAMA/LABA combination therapy is recommended over LABA or LAMA monotherapy in COPD patients with dyspnea or exercise intolerance (strong recommendation, moderate certainty of evidence) |
*, from the GOLD document 2019, the recommendations for medical treatment are divided into initial treatment and follow-up. GOLD groups of patients are not considered in the medical treatment recommendation during follow-up. LAMA, long-acting muscarinic antagonist; LABA, long-acting β agonist; COPD, chronic obstructive pulmonary disease; GesEPOC, Spanish COPD Guideline; NICE, National Institute for Health and Care Excellence; ATS, American Thoracic Society.
Guidelines published by other organizations also include recommendations regarding dual bronchodilator therapy. The latest Spanish COPD Guideline (GesEPOC) (39,40) was published in 2017. COPD patients were divided into low-risk and high-risk groups and four phenotypes: non-exacerbated phenotype, mixed COPD-asthma phenotype, exacerbated emphysema phenotype and exacerbated chronic bronchitis. In this version of the GesEPOC, LAMA/LABA is recommended in low-risk patients who remain symptomatic under monotherapy, and in high-risk patients of non-exacerbated phenotype, exacerbated with emphysema phenotype, and exacerbated with chronic bronchitis phenotype (same phenotypes mentioned in 2014 guideline) as initial treatment (39,40).
The National Institute for Health and Care Excellence (41) guidelines also updated their recommendations regarding the use of fixed-dose dual bronchodilator therapy for the first time in 2018. LAMA/LABA is recommended for COPD patients (confirmed by spirometry) who have no features suggesting asthma or steroid responsiveness, and remain breathless or have exacerbations despite having used or been offered treatment for tobacco dependence (if smoke), optimized nonpharmacological management, relevant vaccinations, and a short-acting bronchodilator (41). The American Thoracic Society (ATS) published clinical practice guidelines for the pharmacological management of COPD in 2020 (42), and the details of the recommendations regarding LAMA/LABA treatment for stable COPD patients were updated for the first time. They recommended the use of LAMA/LABA dual therapy in COPD patients with dyspnea or exercise intolerance. In general, LAMA/LABA dual therapy is increasingly recommended in the guidelines as more clinical evidence becomes available, especially for patients with more severe disease and those who still have symptoms on monotherapy with bronchodilators.
LAMA/LABA combination therapy in China
Research in China
The efficacy and safety of LAMA/LABA FDCs in the local population were evaluated by summarizing the research data from current trials involving Chinese COPD patients. The efficacy of treatment was discussed from five perspectives, namely, lung function, health status, dyspnea, exacerbations, and safety, including tolerance of the treatment and adverse events. Details on the available evidence are shown in Table 4.
Table 4
| Study | Population | Design | Comparators | Reported outcomes |
|---|---|---|---|---|
| GLY/IND | ||||
| Zhong et al., 2015 (LANTERN) | 744 Chinese patients with moderate to severe COPD with a history of |
26-week MC, RCT, DB, double-dummy, PG study | SFC 50/500 μg b.i.d. | Lung function: trough FEV1, FEV1 AUC0–4h at day 1 and week 26, peak FEV1 at day 1 and week 26, peak FVC (taken over the first 4 hours) was significantly greater with GLY/IND. Health status: the improvements in the SGRQ total scores and total CAT scores in 2 groups are similar. Dyspnea: the improvements in the TDI focal score in 2 groups are similar. Exacerbations: annual moderate or severe exacerbations rate was significantly lower in GLY/IND group than in SFC group. Safety and tolerance: fewer AEs in the patients treated with GLY/IND. The total number of AEs leading to hospitalization in SFC group was almost double of that in GLY/IND group |
| Wedzicha et al., 2017 (FLAME) | 510 Asian patients with moderate to very severe COPD and ≥1 exacerbation in the previous year (316 from the Chinese patients) | 52-week, MC, RCT, DB, double-dummy, PG, noninferiority, actively controlled study | SFC 50/500 µg b.i.d. | Lung function: improvement in pre-dose trough FEV1 was significantly greater with GLY/IND than with SFC. Health status: over the 52-week treatment period, the improvement in the SGRQ-C total score was greater with GLY/IND than with SFC. Dyspnea: –. Exacerbations: GLY/IND significantly reduced the rate of moderate or severe exacerbations, and prolonged the time to the first moderate or severe exacerbation. Safety and tolerance: the incidences of AEs in 2 groups were similar. The pneumonia incidence was numerically lower in the GLY/IND group |
| UMEC/VI | ||||
| Zheng et al., 2015 | 739 patients with an established clinical history of COPD (467 Chinese patients) | 24-week, phase III, MC, RDBPC, PG study | UMEC/VI |
Lung function: trough FEV1 and trough FVC on day 169 were both significantly greater in the UMEC/VI groups vs. placebo. Health Status: UMEC/VI groups had a significantly improved SGRQ total score on day 84 and clinically meaningful improvements in the mean CAT scores vs. placebo from baseline on day 168. Dyspnea: TDI scores for the UMEC/VI groups were significantly greater vs. placebo on day 168. Exacerbations: UMEC/VI 125/25 μg reduced the risk of exacerbation vs. placebo, although the same effect was not observed for UMEC/VI 62.5/25 μg. Safety and Tolerance: Incidences of AEs were similar across treatment groups |
| TIO/OLO | ||||
| Wang et al., 2020 | 12 Chinese patients with moderate to severe COPD | 3-week, single site, OL, phase Ib clinical study | – | Lung function: –. Health status: –. Dyspnea: –. |
| Bai et al., |
548 Chinese patients with moderate to very severe COPD | 52-week, DB, PG, actively controlled, RCT, phase III studies (TONADO 1+2) | TIO 5 μg, OLO |
Lung function: trough FEV1 and adjusted mean FEV1 AUC0–3 h were significantly greater with TIO/OLO than with TIO or OLO monotherapy. Health status: TIO/OLO significantly improved SGRQ scores compared with OLO monotherapy. Dyspnea: –. Exacerbations: –. Safety and tolerance: the safety profile of TIO/OLO was comparable with that of monotherapy over 52 weeks. AEs were not increased in the TIO/OLO group compared with the monotherapy groups |
| GFF | ||||
| Chen |
466 Chinese patients with moderate to very severe COPD | RDBPC, PG, phase III study (PINNACLE-4) | GP 18 µg, FF |
Lung function: the change from baseline in the pre-dose trough FEV1 at week 24 was significantly greater with GFF than monotherapy or placebo. Health status: the estimated difference from baseline in the SGRQ score at week 24 was lower with GFF than placebo. Dyspnea: TDI focal score over 24 weeks showed a clinically meaningful improvement with GFF compared to the placebo. Exacerbations: the risk of a moderate or severe exacerbation was numerically lower in the GFF group than in the GP, FF, and placebo groups. GFF reduced CID. Safety and tolerance: AE rates were similar across all active treatment groups and slightly higher in the placebo group |
LAMA, long-acting muscarinic antagonist; LABA, long-acting β agonist; FDC, fixed-dose combination; GLY/IND, glycopyrronium/indacaterol; UMEC/VI, umeclidinium/vilanterol; GFF, glycopyrrolate/formoterol fumarate; TIO/OLO, tiotropium/olodaterol; ACL/FF, aclidinium/formoterol; TIO/FF, tiotropium/formoterol; AEs, adverse events; CAT, COPD Assessment Test; CID, clinically important deterioration; FEV1, forced expiratory volume in 1 second; FEV1 AUC0–4h, FEV1 area under the curve from 0–4 h; FVC, forced vital capacity; TDI, Transition Dyspnea Index; SFC, salmeterol/fluticasone; SGRQ, St. George’s Respiratory Questionnaire; SGRQ-C, SGRQ for COPD; vs., versus; –, no related content; MC, multi-centre; DB, double blind; RCT, randomized controlled trial; PG, parallel group; MNC, multinational; OL, open-label; RDBPC, randomized, double-blind, placebo-controlled.
Lung function
A range of lung function measures show improvements with LAMA/LABA FDCs compared with placebo, monotherapy and the combination of salmeterol and fluticasone (SFC) (43-47). Chen et al. (46) found that the trough FEV1 was significantly better in patients receiving GFF than glycopyrronium (GP), formoterol (FF), and placebo (least-squares mean differences: 98, 104, and 173 mL, respectively (all P≤0.0001). Zhong et al. (43) found that GLY/IND was superior to SFC regarding the improvement in the trough FEV1. They also found a significant improvement in the standardized FEV1 area under the curve (AUC) from 0 to 4 hours in the GLY/IND group at week 26 compared with the SFC group.
Health status
LAMA/LABA FDCs had beneficial effects on the health status of patients, as assessed by SGRQ. In an Asian subgroup analysis from the FLAME study (including data from Chinese patients) conducted by Wedzicha et al. (48), they found that GLY/IND significantly improved the SGRQ for COPD scores (SGRQ-C score; P=0.006) compared with SFC. Additionally, Zhong et al. (43) found similar results in a Chinese subgroup analysis of the data from the LANTERN study. TIO/OLO also significantly improved SGRQ scores compared with OLO monotherapy in Chinese patients (32.729 and 37.202, respectively).
Dyspnea
As a reflection of the ability of LAMA/LABA FDCs to improve lung function in patients, they also demonstrated beneficial effects on dyspnea. UMEC/VI and GFF significantly improved the TDI total score compared with the placebo (47,49); similarly, the improvement in the TDI focal score in the GLY/IND treatment group was greater than that in the SFC treatment group in Chinese patients (43).
COPD exacerbations
A few studies in Chinese patients have reported results on COPD exacerbations. In the Asian subgroup analysis of the FLAME study, which included moderate to very severe COPD patients with ≥1 exacerbation in the previous year, GLY/IND significantly reduced the moderate or severe exacerbation rate compared with SFC; additionally, GLY/IND prolonged the time to the first moderate or severe exacerbation, with a 23% reduction in the risk (48). In the LANTERN study, Zhong et al. (43) found that the annual moderate or severe exacerbation rate of patients in the GLY/IND treatment group was significantly lower than that in the SFC treatment group. Compared with SFC, GLY/IND significantly reduced the moderate or severe exacerbation rate by 31% (P=0.048). Chen et al. (46) reported that in the Chinese subpopulation in the PINNACLE-4 study, the moderate or severe COPD exacerbation risk in the GFF treatment group was numerically lower than those in the GP, FF, and placebo groups; GFF reduced the risk of clinically important deterioration compared to monotherapies and the placebo.
Safety and tolerability
The individual LABA and LAMA components of LAMA/LABA FDCs have well-characterized safety profiles (50). To date, LAMA/LABA FDCs have been found to have feasible overall safety and tolerability profiles in studies including Chinese patients (duration of at least 24 weeks, up to 52 weeks). The rates of adverse events needing emergency treatment across treatment groups (GFF vs. GLY and FF) were similar or slightly higher in the placebo MDI group (46). Compared with TIO or OLO monotherapy, more adverse events were not reported by the TIO/OLO group (45). The pneumonia incidence in the GLY/IND group was threefold lower than that in the SFC group (0.8% for GLY/IND vs. 2.7% for SFC) (43). Wang et al. (49) also reported that TIO/OLO was well tolerated in the Chinese population.
Chinese guidelines and consensus
The first Chinese COPD guidelines were published by the COPD Group of the Respiratory Disease Branch of the Chinese Medical Association in 1997—Chronic obstructive pulmonary disease (COPD) diagnosis and treatment specifications (draft) (51)—and have been updated iteratively ever since. LAMA/LABA FDCs were mentioned in the guidelines before they entered the Chinese market. The version revised in 2013 (Chinese Guidelines for the Diagnosis and Treatment of COPD) (52) mentioned that the combined use of bronchodilators with different mechanisms of action and durations could enhance bronchial relaxation and reduce adverse reactions; the combined application of β2 agonists and anticholinergic medicines could further improve patients’ lung function and health status. There was no clear statement regarding which group of patients or under which circumstances dual bronchodilator therapy was recommended. In the latest Chinese COPD guideline, Guideline for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021) (53), LAMA/LABA is recommended to specific patient group. For GOLD B and D Group patients with CAT (COPD assessment test) scores >20, LAMA/LABA is recommended to use, especially in GOLD D Group, it is as the first choice; During follow-up, patients who still have dyspnea, exercise limitations or exacerbations on monotherapy should use dual bronchodilators. Dual bronchodilators or LABA/ICS dual therapy were recommended for this group of patients to reduce exacerbations. Besides, Patients on triple therapy should downgrade to LAMA/LABA. when with any inappropriate ICS use indications. Considering their relatively low price and excellent bronchodilation effect, LAMA/LABA combinations were recommended as the first choice for this group of patients.
Guidelines have been issued by other organizations in more recent years, in which detailed statements describing the benefits of LAMA/LABA combination therapy and the recommended populations have been included. The Guideline for the Primary Care of Chronic Obstructive Pulmonary Disease: Practice Version (published in 2018) (54) points out that LAMA/LABA FDCs are superior to monotherapy regarding improving symptoms, improving the FEV1, and reducing acute exacerbations (54). Patients with mild or moderate airflow limitation whose symptoms are not controlled by short-acting bronchodilators or with a long-acting bronchodilators or patients at high exacerbations risk may consider LAMA/LABA (54). In addition, in the Clinical Practice Guideline for the Diagnosis and Management of Chronic Obstructive Pulmonary Disease in Elderly Patients (published in 2020) (55). For patients with mild or moderate airflow limitation whose symptoms are not controlled with monotherapy, LAMA/LABA is still recommended. Besides, for patients with severe or very severe airflow limitation or with a high exacerbations risk, LAMA/LABA dual therapy is recommended as the first choice. Details of the change of guidelines mentioned above are shown in Table 5.
Table 5
| Guideline | Year | Recommendation |
|---|---|---|
| Chinese Guidelines for the Diagnosis and Treatment of COPD | 2013 | There is no clear statement regarding for which group of patients or under which circumstances dual bronchodilator therapy is recommended |
| Guidelines for the primary care of chronic obstructive pulmonary disease: practice version | 2018 | Patients with mild or moderate airflow limitation (FEV1 ≥50%): if the symptoms are not controlled by short-acting bronchodilators or with a LAMA or a LABA, combination therapy, including LABA/ICS and LAMA/LABA, is recommended. Patients at high risk of acute exacerbations (patients with severe airflow obstruction (FEV1<50%), multiple symptoms, or frequent acute exacerbations) should use combination therapy, including LABA/ICS and LAMA/LABA |
| Clinical practice guidelines for the diagnosis and management of chronic obstructive pulmonary disease in elderly patients | 2020 | For patients with mild or moderate airflow limitation whose symptoms are not controlled with monotherapy and patients at high risk of acute exacerbations, LAMA/LABA dual therapy is recommended. LAMA/LABA dual therapy is recommended as the first choice in patients with severe or very severe airflow limitation or with a high risk of acute exacerbations |
| Guidelines for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021) | 2021 | GOLD B Group: for patients with CAT scores >20, LAMA/LABA dual therapy may be considered. GOLD D Group: LAMA or LAMA/LABA dual therapy or ICS/LABA dual therapy or ICS/LAMA/LABA triple therapy can be chosen according to the patient’s condition. For patients with CAT scores >20, dual bronchodilator therapy is recommended as the first choice. During follow-up: patients who still have dyspnea, exercise limitations or acute exacerbations on LAMA or LABA monotherapy should use dual bronchodilators. Patients on triple therapy should downgrade to dual bronchodilators when any indications of inappropriate ICS use are found, including the use of ICS in patients without a history of exacerbations, no response to ICS, and ICS-related adverse events such as recurrent pneumonia or mycobacterial infection |
LAMA, long-acting muscarinic antagonist; LABA, long-acting β agonist; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; ICS, inhaled corticosteroid.
The compilation of the Chinese COPD guidelines has mainly been based on the actual situation in China and the progress of COPD research in China and abroad. Ongoing clinical trials are providing a wealth of evidence on the effects of LAMA/LABA FDCs, including evidence in Chinese COPD patients. We can also see that this type of evidence has accumulated over time, leading to the increase in the importance of LAMA/LABA FDCs for the treatment of COPD in the Chinese guidelines. In addition to the clinical evidence, the clinical characteristics of Chinese patients also support the increasing importance of dual bronchodilators for the treatment of COPD. Most Chinese COPD patients live in rural areas; due to the relatively inadequate medical resources in rural areas, these patients often come to the hospital when their symptoms become obvious (56,57). This leads to relatively more severe respiratory symptoms in COPD patients diagnosed in China. Correspondingly, awareness of the role of LAMA/LABA dual therapy in the management of COPD is evolving due to its effect on relieving respiratory symptoms, improving lung function, and reducing acute attacks. It is worth mentioning that there is a gap between the recommendations in previous Chinese guidelines and the GOLD documents. For instance, LABA/ICS dual therapy was too widely recommended in previous Chinese guidelines, while the recommendations for LAMA/LABA dual therapy were limited compared with those in the GOLD document, which was published at the same time. In reality, there remains a problem with the excessive prescription of ICS to Chinese COPD patients, and the use of bronchodilators is still insufficient. In the latest version of Guidelines for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021), the recommendations regarding ICS are consistent with those in the GOLD 2021 document, while there are differences in the recommendations regarding LAMA/LABA FDCs. In the Chinese guidelines, LAMA/LABA FDCs are recommended for GOLD group B patients with CAT scores >20, which is not the recommendation in the GOLD document. This difference is an adjustment due to the more severe dyspnea symptoms in Chinese COPD patients and sufficient clinical evidence regarding LAMA/LABA dual therapy.
Availability and affordability of LAMA/LABA dual therapy in China
In the “Healthy China 2030” report (58), COPD was listed as a key chronic disease that needed support. For instance, after GLY/IND FDCs were approved for the Chinese market, it took only one year for GLY/IND to be included in the medical insurance catalog, which reflected the preferential policy. Current medical policy gives particular attention to chronic respiratory diseases, such as COPD; therefore, a series of interventions have been introduced, including but not limited to introducing new medicines, adding new medicines to the medical insurance catalog, and setting up convenient outpatient clinics. In addition, in recent years, Chinese medical insurance system has rapidly improved. The coverage rate of Chinese medical insurance was less than 50% in 2005, and it increased to 95% by 2011 (59,60). Under the new medical insurance system, a certain percentage of the expenses incurred during hospitalization and the expenses for maintenance treatment after discharge can be reimbursed. However, not all medicines are covered by medical insurance, and only those included in the medical insurance catalog are eligible for reimbursement. As mentioned above, there are four LAMA/LABA combinations on the Chinese market, GLY/IND, GFF, and UMEC/VI were included in the new version of the medical insurance catalog. There are regional differences in the reimbursement rates for Class B drugs, ranging from 90% to 70%.
In addition, there are differences in medical treatment among regions, between urban and rural areas, and even between hospitals (14). Chinese medical insurance reimbursement system mainly reimburses a certain percentage of medical expenses, as mentioned above; therefore, patients need to cover a fixed proportion of the cost of medications, which makes patients more sensitive to the cost-effectiveness of the drug. A previous study in other countries on the cost-effectiveness of LAMA/LABA FDCs compared to SFCs and the individual components showed that LAMA/LABAs are cost-minimizing (61,62). A study in China also showed a similar result. Gong et al. (63) compared the cost-effectiveness of GLY/IND with the cost-effectiveness of SFC and tiotropium. The results showed that from the payer’s perspective, GLY/IND is cost-effective for the treatment of stable COPD, and GLY/IND is more efficient and less expensive than SFC. From an economic perspective, medical expenses include not only medication expenses but also those associated with the use of other health resources. When patients experience worsening symptoms leading to hospitalization, they incur greater medical expenses. Therefore, effective daily disease control can relieve the burden on the health system and the medical insurance fund. Moreover, on the Chinese market, the cost of LAMA/LABA FDCs is clearly lower than the combination of two separate single bronchodilators inhalers of the same dose, indicating that LAMA/LABA FDCs is a more affordable option than free combination of LAMA/LABA. LAMA/LABA FDCs have significant overall advantages in clinical practice, including improvements in dyspnea symptoms and lung function and a reduction in acute exacerbations. These advantages have been confirmed in major clinical studies, such as SPARK, FLAME, LANTERN and other studies, as summarized previously.
Medication is the key to COPD management, and clinicians and health commission experts remain concerned with the cost-effectiveness of medical treatments. The newly published pharmacoeconomics of LAMA/LABA FDCs in COPD patients confirmed its superior cost-effectiveness. In the future, further stratified and real-world research needs to be conducted to establish a pharmacoeconomic evaluation system that is more suitable for the Chinese population.
Discussion
COPD is the most common chronic airway disease, and it is also a disease that is a focus of prevention and treatment in the Healthy China 2030 Action Plan. Chinese COPD guidelines play an essential guiding role in clinical practice.
As emphasized above, Chinese COPD patients are characterized by severe respiratory symptoms, and patients who visit the outpatient clinic or emergency department generally have clear symptoms. Accumulated experimental and clinical evidence in recent years has also confirmed that LAMA/LABA FDCs can relieve dyspnea, improve lung function, and reduce acute exacerbations. LAMA/LABA FDCs are more convenient than free combinations of LAMA/LABA, which improves adherence (26). Moreover, on the Chinese market, LAMA/LABA FDCs is more affordable than the LAMA/LABA in two separate inhalers of the same dose. The role of LAMA/LABA, SFC, and triple therapy in treatment has become clearer as research progressing. As we can observed in guidelines, the recommendation of combination inhalers with ICS (SFC and triple therapy) have been limited to patients with frequent exacerbators, COPD/asthma overlapping, as these patients can benefit more considering both the effective and adverse events. Current clinical trials show that LAMA/LABA is more efficient than monotherapies and SFC. Given the severity of respiratory symptoms in Chinese patients, LAMA/LABA is an appropriate therapeutic option that is predicted to be applied in a larger number of patients, particularly those whose symptoms are not controlled by current treatment.
Based on this, the importance of dual bronchodilator therapy has increased in the Chinese guidelines. In the latest Chinese guidelines (updated in 2021), LAMA/LABA combinations were recommended for patients in GOLD groups B and D, while they were only recommended in GOLD group D in the 2021 version of the GOLD document. In addition to more severe respiratory symptoms in Chinese COPD, it is noteworthy that although most Chinese COPD patients undergo pulmonary function tests, as they are routinely administered in hospitals, there are no pulmonary function indicators in the current GOLD documents and Chinese guidelines. In addition, the prognosis and treatment response are heterogeneous in COPD patients. Therefore, establishing a Chinese clinical pathway that includes an assessment and management algorithm that matches the clinical characteristics in Chinese patients and classifies the phenotypic characteristics of COPD according to a suitable system is needed.
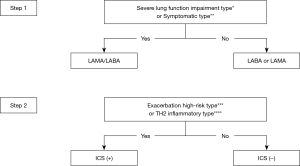
The current clinical pathway in the 2021 version of the Chinese guidelines is consistent with the GOLD document. This clinical pathway is quite robust, but it also has some disadvantages: it is somewhat complicated for use in real-world clinical practice, and it is not tailored to Chinese patients and doctors. We focused on the information combined from the current guidelines and the characteristics of Chinese patients. For newly diagnosed COPD patients, the suggested classification divides them into four phenotypes: symptomatic type [CAT>10 or Modified Medical Research Council Dyspnea Scale (mMRC) ≥2]; severe lung function impairment type (FEV1 <50%); TH2 inflammatory type [blood eosinophil (EOS) ≥300/µL]; and high-risk exacerbation type (≥2 moderate exacerbations or ≥1 leading to hospitalization). The initial treatment of patients is determined in two steps. The first step is to measure the lung function (according to the airflow limitation classification) and symptoms of the patient. LAMA/LABA FDCs are recommended for patients with symptoms and/or severe lung function impairment. The second step is to evaluate the patient’s exacerbation history and EOS level. If the patient is at high risk of TH2 inflammation or exacerbation, ICS is recommended in addition to the current treatment with bronchodilator(s). More details are shown in Figure 1.
Conclusions
COPD is a major contributor to the burden of chronic respiratory disease in China, and the symptoms experienced by Chinese patients are relatively more severe. LAMA/LABA FDCs are a group of efficient, quick-acting and long-lasting bronchodilator combinations with good safety. Based on the characteristics of Chinese COPD patients, we can conclude that LAMA/LABA FDCs are a suitable choice to reduce symptoms and improve the QoL and economic outcomes of patients. Due to the complexity and heterogeneity of COPD and the fact that LAMA/LABA FDCs have been on the Chinese market and covered by China’s medical insurance for less than 5 years, there are still some problems to be addressed. For example, how do LAMA/LABA FDCs perform in the treatment of patients in the real world? How can we develop an assessment and management algorithm for COPD patients that can lead to the selection of more accurate and suitable treatment plans for Chinese patients? Which biomarkers should be used to guide individualized diagnosis and treatment? Many questions could not be addressed within the scope of this article, and we look forward to more studies in the future that can guide the further improvement of the treatment of Chinese COPD patients.
Acknowledgments
We are indebted to specialists from the Shanghai COPD Alliance for support, including reviewing the literature and providing revision suggestions. In addition, we are indebted to Hanhui Pharmaceutical Co., Ltd. for professional medical support.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-961
Peer Review File: Available at https://dx.doi.org/10.21037/jtd-21-961
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-961). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- O'Donnell DE, Laveneziana P. The clinical importance of dynamic lung hyperinflation in COPD. COPD 2006;3:219-32. [Crossref] [PubMed]
- Donaldson GC, Seemungal TA, Patel IS, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest 2005;128:1995-2004. [Crossref] [PubMed]
- Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018;391:1706-17. [Crossref] [PubMed]
- Zhu B, Wang Y, Ming J, et al. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis 2018;13:1353-64. [Crossref] [PubMed]
- Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin 2009;25:2043-8. [Crossref] [PubMed]
- Miller J, Edwards LD, Agustí A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med 2013;107:1376-84. [Crossref] [PubMed]
- Zha Z, He Y, Xu W, et al. Effects of smoking on chronic obstructive pulmonary disease and respiratory symptoms. Chin J Dis Control Prev 2020;24:46-51.
- Sun L, Chen Y, Chang C, et al. Present status of medical treatment for patients with chronic obstructive pulmonary disease based upon different severity classifications. Zhonghua Yi Xue Za Zhi 2015;95:570-6. [PubMed]
- Ding B, Small M, Bergström G, et al. COPD symptom burden: impact on health care resource utilization, and work and activity impairment. Int J Chron Obstruct Pulmon Dis 2017;12:677-89. [Crossref] [PubMed]
- Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med 2007;176:753-60. [Crossref] [PubMed]
- Xiao T, Chen X, Wang N, et al. Study on the situation of drug use in patients with chronic obstructive pulmonary diseases in the Chinese communities of large cities. Zhonghua Liu Xing Bing Xue Za Zhi 2017;38:142-6. [PubMed]
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [Crossref] [PubMed]
- Zhang Y, Wang N, Fan J, et al. Analysis in medication treatment and its related factors among patients with chronic obstructive pulmonary disease aged 40 years or older in China, 2014-2015. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41:678-84. [PubMed]
- He Q. Current Situation of Diagnosis and Treatment of COPD in Rural Primary Hospitals of China. Chin J Respi Crit Care Med 2014;13:5-9.
- Cui Y, Dai Z, Luo L, et al. Classification and treatment of chronic obstructive pulmonary disease outpatients in China according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017: comparison with GOLD 2014. J Thorac Dis 2019;11:1303-15. [Crossref] [PubMed]
- Mirza S, Clay RD, Koslow MA, et al. COPD Guidelines: A Review of the 2018 GOLD Report. Mayo Clin Proc 2018;93:1488-502. [Crossref] [PubMed]
- Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol (1985) 2006;101:971-85. [Crossref] [PubMed]
- Cazzola M, Page CP, Calzetta L, et al. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev 2012;64:450-504. [Crossref] [PubMed]
- Alagha K, Palot A, Sofalvi T, et al. Long-acting muscarinic receptor antagonists for the treatment of chronic airway diseases. Ther Adv Chronic Dis 2014;5:85-98. [Crossref] [PubMed]
- Cazzola M, Matera MG, Lötvall J. Ultra long-acting beta 2-agonists in development for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs 2005;14:775-83. [Crossref] [PubMed]
- Calzetta L, Matera MG, Rogliani P, et al. Dual LABA/LAMA bronchodilators in chronic obstructive pulmonary disease: why, when, and how. Expert Rev Respir Med 2018;12:261-4. [Crossref] [PubMed]
- Cazzola M, Matera MG. POINT: Should LAMA/LABA Combination Therapy Be Used as Initial Maintenance Treatment for COPD? Yes. Chest 2018;154:746-8. [Crossref] [PubMed]
- Cazzola M, Calzetta L, Segreti A, et al. Translational study searching for synergy between glycopyrronium and indacaterol. COPD 2015;12:175-81. [Crossref] [PubMed]
- Pelaia G, Maselli R, Matera MG. Treatment of chronic obstructive pulmonary disease by dual bronchodilation with coformulation of indacaterol/glycopyrronium. Pharmacology 2014;94:249-58. [Crossref] [PubMed]
- Cazzola M, Calzetta L, Puxeddu E, et al. Pharmacological characterisation of the interaction between glycopyrronium bromide and indacaterol fumarate in human isolated bronchi, small airways and bronchial epithelial cells. Respir Res 2016;17:70. [Crossref] [PubMed]
- de Miguel-Díez J, Jiménez-García R. Considerations for new dual-acting bronchodilator treatments for chronic obstructive pulmonary disease. Expert Opin Investig Drugs 2014;23:453-6. [Crossref] [PubMed]
- Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2017;12:907-22. [Crossref] [PubMed]
- Calzetta L, Rogliani P, Matera MG, et al. A Systematic Review With Meta-Analysis of Dual Bronchodilation With LAMA/LABA for the Treatment of Stable COPD. Chest 2016;149:1181-96. [Crossref] [PubMed]
- Calzetta L, Ora J, Cavalli F, et al. Impact of LABA/LAMA combination on exercise endurance and lung hyperinflation in COPD: A pair-wise and network meta-analysis. Respir Med 2017;129:189-98. [Crossref] [PubMed]
- Banerji D, Fogel R, Patalano F. Indacaterol/glycopyrronium: a dual bronchodilator for COPD. Drug Discov Today 2018;23:196-203. [Crossref] [PubMed]
- Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med 2013;1:51-60. [Crossref] [PubMed]
- Vogelmeier C, Zhong N, Humphries MJ, et al. Indacaterol/glycopyrronium in symptomatic patients with COPD (GOLD B and GOLD D) versus salmeterol/fluticasone: ILLUMINATE/LANTERN pooled analysis. Int J Chron Obstruct Pulmon Dis 2016;11:3189-97. [Crossref] [PubMed]
- Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting β-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax 2016;71:15-25. [Crossref] [PubMed]
- Suissa S, Dell'Aniello S, Ernst P. Comparative Effectiveness and Safety of LABA-LAMA vs. LABA-ICS Treatment of COPD in Real-World Clinical Practice. Chest 2019;155:1158-65. [Crossref] [PubMed]
- Vogel-Claussen J, Schönfeld CO, Kaireit TF, et al. Effect of Indacaterol/Glycopyrronium on Pulmonary Perfusion and Ventilation in Hyperinflated Patients with Chronic Obstructive Pulmonary Disease (CLAIM). A Double-Blind, Randomized, Crossover Trial. Am J Respir Crit Care Med 2019;199:1086-96. [Crossref] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD), Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, 2021. Available online: https://goldcopd.org/2021-gold-reports/
- Singh D, Agusti A, Anzueto A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J 2019;53:1900164 [Crossref] [PubMed]
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017;195:557-82. [Crossref] [PubMed]
- Cabrera López C, Casanova Macario C, Marín Trigo JM, et al. Prognostic Validation Using GesEPOC 2017 Severity Criteria. Arch Bronconeumol 2019;55:409-13. (Engl Ed). [Crossref] [PubMed]
- Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish guideline for COPD (GesEPOC). Update 2014. Arch Bronconeumol 2014;50:1-16. [Crossref] [PubMed]
- NICE, Chronic obstructive pulmonary disease in over 16s: diagnosis and management, 2018. Available online: https://www.nice.org.uk/guidance/NG115
- Nici L, Mammen MJ, Charbek E, et al. Pharmacologic Management of Chronic Obstructive Pulmonary Disease. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2020;201:e56-69. [Crossref] [PubMed]
- Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis 2015;10:1015-26. [PubMed]
- Zheng J, Xu JF, Jenkins M, et al. Glycopyrrolate/formoterol fumarate metered dose inhaler for maintenance-naïve patients with chronic obstructive pulmonary disease: a post-hoc analysis of the randomized PINNACLE trials. Respir Res 2020;21:69. [Crossref] [PubMed]
- Bai CX, Tang Y, Xin JB, et al. The efficacy and safety of tiotropium/olodaterol fixed-dose combination in Chinese patients with chronic obstructive pulmonary disease: a pooled subgroup analysis of TONADO 1+2. Zhonghua Jie He He Hu Xi Za Zhi 2019;42:838-44. [PubMed]
- Chen R, Zhong N, Wang HY, et al. Efficacy And Safety Of Glycopyrrolate/Formoterol Fumarate Metered Dose Inhaler (GFF MDI) Formulated Using Co-Suspension Delivery Technology In Chinese Patients With COPD. Int J Chron Obstruct Pulmon Dis 2020;15:43-56. [Crossref] [PubMed]
- Zheng J, Zhong N, Newlands A, et al. Efficacy and safety of once-daily inhaled umeclidinium/vilanterol in Asian patients with COPD: results from a randomized, placebo-controlled study. Int J Chron Obstruct Pulmon Dis 2015;10:1753-67. [Crossref] [PubMed]
- Wedzicha JA, Zhong N, Ichinose M, et al. Indacaterol/glycopyrronium versus salmeterol/fluticasone in Asian patients with COPD at a high risk of exacerbations: results from the FLAME study. Int J Chron Obstruct Pulmon Dis 2017;12:339-49. [Crossref] [PubMed]
- Wang Z, Tadayasu Y, Hu N, et al. Pharmacokinetics and safety of tiotropium+olodaterol 5 µg/5 µg fixed-dose combination in Chinese patients with COPD. Pulm Pharmacol Ther 2020;63:101944 [Crossref] [PubMed]
- Rhee CK, Yoshisue H, Lad R. Fixed-Dose Combinations of Long-Acting Bronchodilators for the Management of COPD: Global and Asian Perspectives. Adv Ther 2019;36:495-519. [Crossref] [PubMed]
- Chronic obstructive pulmonary disease (COPD) diagnosis and treatment specifications (draft). Chin J Tubere Respir Dis 1997;(4).
- C.O.P.D.G. Branch of Respiratory Diseases of Chinese Medical Association. Chinese Guidelines for the Diagnosis and Treatment of COPD (2013 Update). Zhonghua Jie He He Hu Xi Za Zhi 2013;36:255-64.
- Liang ZY, Chen RC. Revision to the guidelines for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021): process and perspective. Zhonghua Jie He He Hu Xi Za Zhi 2021;44:165-6. [PubMed]
- Association CM, House CMJP. Guideline for primary care of chronic obstructive pulmonary disease: practice version (2018). Chin J Gen Pract 2018;17:856-70.
- Clinical practice guideline for diagnosis and management of chronic obstructive pulmonary disease in elderly patients. Zhonghua Liu Xing Bing Xue Za Zhi 2020;43:100-19. [PubMed]
- Zhang Y, Wang N, Fan J, et al. Analysis in medication treatment and its related factors among patients with chronic obstructive pulmonary disease aged 40 years or older in China, 2014-2015. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41:678-84. [PubMed]
- He Q. Current Situation of Diagnosis and Treatment of COPD in Rural Primary Hospitals of China. Chin J Respir Critical Care Med 2014;13:5-9.
- Tan X, Liu X, Shao H. Healthy China 2030: A Vision for Health Care. Value Health Reg Issues 2017;12:112-4. [Crossref] [PubMed]
- Yu H. Universal health insurance coverage for 1.3 billion people: What accounts for China's success? Health Policy 2015;119:1145-52. [Crossref] [PubMed]
- Sun Y, Gregersen H, Yuan W. Chinese health care system and clinical epidemiology. Clin Epidemiol 2017;9:167-78. [Crossref] [PubMed]
- Wilson MR, Patel JG, Coleman A, et al. Cost-effectiveness analysis of umeclidinium/vilanterol for the management of patients with moderate to very severe COPD using an economic model. Int J Chron Obstruct Pulmon Dis 2017;12:997-1008. [Crossref] [PubMed]
- Price D, Keininger D, Costa-Scharplatz M, et al. Cost-effectiveness of the LABA/LAMA dual bronchodilator indacaterol/glycopyrronium in a Swedish healthcare setting. Respir Med 2014;108:1786-93. [Crossref] [PubMed]
- Gong S, Hu H, Zhao K, et al. Cost-Effectiveness of Dual Bronchodilator Indacaterol/Glycopyrronium for COPD Treatment in China. Int J Chron Obstruct Pulmon Dis 2021;16:433-41. [Crossref] [PubMed]


