Perioperative outcomes of upper lobectomy according to preservation or division of the inferior pulmonary ligament
Introduction
Inferior pulmonary ligament division in upper lobectomy is believed to be helpful for reducing free space in the upper thorax, and thus for preventing postoperative complications such as pooling of pleural effusion, empyema, insufficient expansion of the remaining lung, and prolonged air leak (1). Although some textbooks state that this procedure should be performed to release the middle or lower lobes, there is no scientific evidence of the clinical advantage of this procedures (2).
Several studies have discussed the potential association between excessive bronchial displacement and inferior pulmonary ligament division, which can lead to disastrous complications (2-4). The beneficial effects of this procedure are controversial, and its performance during upper lobectomy is largely based on surgeon preference. Upper lobectomy is frequently associated with bronchial kink, and this could worsen postoperative pulmonary function (5,6), but there is a lack of evidence of this relationship.
We analyzed perioperative outcomes including the presence of dead space and changes in bronchial angles between patients with lung cancer who underwent division of the inferior pulmonary ligament during upper lobectomy and those who did not.
Materials and methods
Patients
Between March 2012 and November 2013 a total of 207 patients were underwent lobectomies by a single surgeon (K. Kim) in our institute. Among those, 72 patients who underwent video-assisted thoracic surgery (VATS) surgeries for non-small cell lung cancer (NSCLC) were enrolled in this study. Inclusion criteria were as follows: (I) patients who were diagnosed with NSCLC; (II) patients who had cancers in their right or left upper lobes; (III) patients who underwent VATS surgeries for their cancer lesions.
Patients who underwent open thoracotomy or conversion to open (n=9), patients whose diagnoses were other than NSCLC (n=21), and patients who underwent surgeries on their lower or middle lobes (n=102) were excluded.
Enrolled patients were categorized into two groups: the division group, who underwent division of the inferior pulmonary ligament, and the preservation group, who did not. The amount of chest tube drainage and chest tube duration was measured via retrospective review of medical records. The presence of dead space was detected via postoperative chest X-ray. Change in lower lobe angle was measured on chest CT taken 1 month postoperative. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB number; B-1412/280-112).
Pleural effusion
We checked the number of days that daily chest tube drainage was >200 mL, chest tube duration and the chest X-ray at 1 month postoperatively. The indications for chest tube removal included a total amount of daily chest tube drainage <200 mL and no air leakage.
Expansion of the remaining lung
We used the method described by Matsuoka et al. (2) to check the expansion of the remaining lung. The chest X-rays (posterior-anterior) performed preoperatively at end-inspiration, and 1 month postoperatively were reviewed and compared; thereafter the existence of the dead space at the apex was evaluated.
Change in bronchial angle
We used a previously described method (6) to calculate bronchial angle. We used coronal view CT preoperatively and 1 month postoperatively and measured the angles formed by the main bronchus and the bronchus intermedius on the right side, and by the main bronchus and the lower bronchus on the left side. Bronchial angle change was defined as the angle difference between postoperative and preoperative CT images (Figure 1).
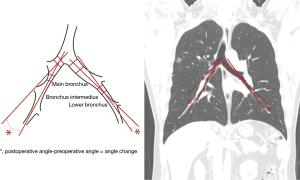
Three-dimensional (3D) reconstruction of chest CT
Reconstruction of 3D chest CT images was performed using a reconstruction program (Lung Quest™, SuperDimension, Inc., Minneapolis, MN, USA) to analyze changes in bronchial diameter and angle more precisely. The same chest CT images we used to measure change in angle were used in analysis. Not all CT images could be reconstructed into 3D images because of the lack of digital information necessary for this program. Chest CT images of 27 patients who underwent right upper lobectomy (19 in the division group and 8 in the preservation group) and 19 who underwent left upper lobectomies (8 in the division group and 11 in the preservation group) were reconstructed and included in analysis. Changes in the diameter of remnant bronchus were measured. Changes in the angle between the main bronchus and the remaining lobar bronchus were measured on coronal and sagittal views (Figure 2).
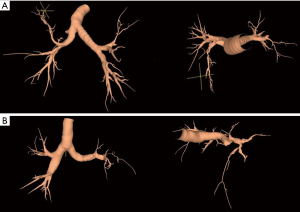
Statistical analysis
We used χ2 and Fisher’s exact test to assess differences in categorical variables. Unpaired Student’s t-test and the Mann-Whitney test were used for discrete and continuous variables. Statistical significance was defined as P values less than 0.05. All statistical analyses were performed using IBM SPSS 20.0 software (IBM Co., Chicago, IL, USA).
Results
Patient and operative characteristics
The mean age of patients was 64.5±9.32 years (range, 35 to 81 years). No significant differences were found between the division and preservation groups in age, sex, smoking history, pathology or staging. Patient characteristics are summarized in Table 1.
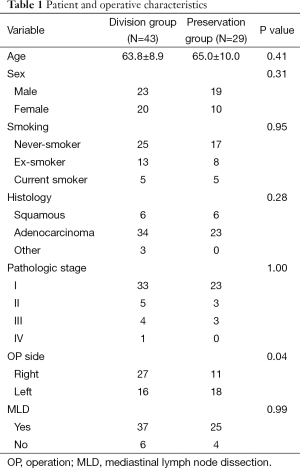
Full table
Pleural effusion
Chest tube duration, duration of chest tube drainage >200 mL, and presence of pleural effusion on follow-up chest X-ray taken 1 month after surgery was not significantly different between the division group and the preservation group (P=0.07, 0.33 and 1.00, respectively). Patients who underwent right and left upper lobectomy did not show significant differences in chest tube duration, duration of chest tube drainage >200 mL, or pleural effusion on postoperative chest X-rays (Table 2).
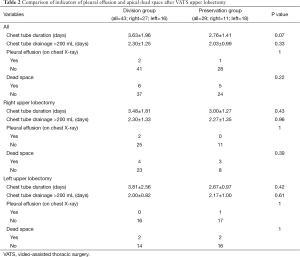
Full table
Expansion of the remaining lung
The presence of apical dead space was evaluated on chest X-rays taken one month after surgery and was observed in 11 patients, but there was no significant difference between division and preservation groups (6 and 5 patients, respectively, P=0.22). Right and left upper lobectomy groups also showed similar apical dead space presence (right, division group: 4, preservation group: 3, P=0.39) (left, division group: 2, preservation group: 2, P=1.00) (Table 2).
Change in bronchial angle in chest X-rays
Bronchial angle change in the division group was 64.1±17.1˚ and that in the preservation group was 65.4±24.0˚, with no significant difference between the groups (P=0.74). In right upper lobectomy patients, the change in bronchial angle in the division group was 65.4±16.4˚ and that in the preservation group was 59.1±18.0˚, with no significant difference between groups (P=0.31). In left upper lobectomy patients, the change in bronchial angle in the division group was 59.4±23.6˚ and that in the preservation group was 71.2±22.7˚, with no significant difference between groups (P=0.15) (Table 3).

Full table
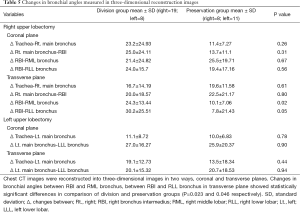
Change in bronchial diameter and angle in 3D reconstruction images
No significant differences were found between the division and preservation groups in RML and LLL bronchial diameter change (P=0.72 and 0.12, respectively). There were also no significant differences in the change in the left main bronchus and left lower lobar (LLL) bronchus angle (0.90 on the coronal view and 0.94 on the sagittal view). Changes in right main bronchus and bronchus intermedius angle were not significantly different in the coronal or transverse plane (P=0.31 and 0.80, respectively); however, there were significant differences in the change in the bronchus intermedius and remnant lobar bronchi angle [P=0.02 for the right middle lobar (RML) bronchus and P=0.046 for the right lower lobar (RLL) bronchus]. The results are summarized in Tables 4,5.

Full table
Full table
Discussion
Although major pulmonary resection is essential for lung cancer cure, sometimes it causes inevitable complications resulting from reduced lung volumes. Division of the inferior pulmonary ligament during upper lobectomy has been performed based on the belief that it can prevent complications caused by free space such as pleural effusion, empyema, and dead spaces (1,2).
However, some surgeons suspect that this procedure could lead to postoperative complications with poor or even fatal outcomes (1,3,4), including bronchial stenosis and bronchial obstruction, resulting from kinking of the bronchus (1,3). The inferior pulmonary ligament may secure the lower lobes; therefore, preservation of this structure could prevent upward displacement of the remaining lower lobe following upper lobectomy, which otherwise could lead bronchial kinking, bronchial stenosis or bronchial obstruction (6).
Decisions regarding inferior pulmonary ligament division tend to be largely based on physician experience because of the lack of research-based evidence. Surgeons who are in favor of this procedure think that it can prevent the formation of apical dead space and pooling of pleural effusion. However, Matsuoka et al. (2) reported that division of the inferior pulmonary ligament does not significantly decrease the dead space ratio on chest X-ray in either right or left upper lobectomy. Our study showed similar results. We compared the presence of apical dead space in chest X-rays taken one month after surgery, and found no significant difference between the division group and the preservation group in both right and left upper lobectomy.
There have been no studies to date that have reported the preventative effects of inferior pulmonary ligament division on the pooling of pleural effusion. In our study, we examined chest tube duration, duration of chest tube drainage >200 mL and the presence of pleural effusion on postoperative chest X-rays to evaluate the pooling of pleural effusion. We found no significant difference in these aspects between the division and preservation groups. Looking at all patients, chest tube duration was longer in the division group, but this difference was not statistically significant. This may be due to our hospital’s recent practice of performing earlier pleurodesis for postoperative air leakage, as the duration of chest tube placement is determined not only by the amount of drainage, but also by the presence of air leakage. Therefore, we believe that the duration of chest tube drainage >200 mL is more important than chest tube duration when analyzing pooling of pleural effusion.
Surgeons who prefer to preserve the inferior pulmonary ligament think that this is an effective method of preventing complications such as bronchial stenosis and bronchial obstruction related to kinking rotation of the remaining bronchi after ligament division. Preservation of the inferior pulmonary ligament may also suppress upward deviation of the remaining lobe (1). In our study, we measured the angle between the main bronchus and the remaining bronchus (6). The advantage of this method is that it is a simple and quick method of measurement on chest CT. However, a limitation of this method is that it cannot confirm whether actual bronchial stenosis or obstruction has occurred. Nevertheless, a key cause of bronchial stenosis and obstruction is excessive bronchial kinking, so the measurement of bronchial angulation could be relevant.
When we measured changes in bronchial angles using the same methods used in Matsuoka’s study; we did not find any significant differences between the division and preservation groups. This might be due to the fact that the degree of bronchial angulation is affected not only by division of the inferior pulmonary ligament, but also by hilar release from lymph node dissection (1,7).
We found significant differences in the change in bronchus intermedius and remnant right lobe angle when we reconstructed chest CT images into 3D images. The changes in bronchus intermedius and RML bronchus angle and in bronchus intermedius and RLL bronchus angle were significantly different between the division and preservation groups when measured in the transverse plane. This finding may suggest that inferior pulmonary ligament division could cause displacement of remnant bronchus, thus leading to bronchial kinking or stenosis.
There are some limitations to this study. First, this study is a retrospective study with a small number of subjects. Second, we did not compare the apical dead space ratio, but, rather, the existence of apical dead space. We may have observed significant differences between groups if we analyzed the apical dead space ratio. However, when calculating the apical dead space ratio it is important that chest X-rays are taken at the exact end of inspiration, and inconsistencies may lead to variation between individuals (8). Other factors such as postoperative pain can limit full inspiration, so we believe that analysis of the presence of apical dead space is a suitable alternative. Finally, although we performed 3D reconstruction using chest CT data, this could not be achieved for all patients. Some chest CT data could not be reconstructed in 3D form, due to a lack of sufficient data necessary for reconstruction.
We detected a possible association between complications caused by bronchial over-dislocation and inferior pulmonary ligament division in upper lobectomy. Because some loss of pulmonary function is inevitable during the treatment of lung cancer, careful consideration of surgical details such as inferior pulmonary ligament division is needed to avoid additional functional loss (9).
The limitation of this study is that it is retrospective research, and that the division of inferior pulmonary ligament was performed at the surgeon’s own decision. We have no exact standard for the division or preservation of inferior pulmonary ligament. Therefore, there might exist selection bias for the decision whether preservation or not. More precisely designed randomized controlled trials should be proceeded to demonstrate more accurate relationship between the complications and the division of inferior pulmonary ligaments.
Conclusions
In conclusion, there is no evidence of advantages of inferior pulmonary ligament division. Rather, we discovered an association between this procedure and excessive dislocation of remnant bronchi. The potential disadvantages of division of the inferior pulmonary ligament during upper lobectomy in lung cancer patients should be carefully considered.
Acknowledgements
Funding: This work was supported by Research Fund of Seoul National University College of Medicine Education Research Foundation (grant number: 800-20130174).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Usuda K, Sagawa M, Aikawa H, et al. Do Japanese thoracic surgeons think that dissection of the pulmonary ligament is necessary after an upper lobectomy? Surg Today 2010;40:1097-9. [PubMed]
- Matsuoka H, Nakamura H, Nishio W, et al. Division of the pulmonary ligament after upper lobectomy is less effective for the obliteration of dead space than leaving it intact. Surg Today 2004;34:498-500. [PubMed]
- Khanbhai M, Dunning J, Yap KH, et al. Dissection of the pulmonary ligament during upper lobectomy: is it necessary? Interact Cardiovasc Thorac Surg 2013;17:403-6. [PubMed]
- Terzi A, Furlan G, Magnanelli G, et al. Chylothorax after pleuro-pulmonary surgery: a rare but unavoidable complication. Thorac Cardiovasc Surg 1994;42:81-4. [PubMed]
- Seok Y, Cho S, Lee JY, et al. The effect of postoperative change in bronchial angle on postoperative pulmonary function after upper lobectomy in lung cancer patients. Interact Cardiovasc Thorac Surg 2014;18:183-8. [PubMed]
- Ueda K, Tanaka T, Hayashi M, et al. Clinical ramifications of bronchial kink after upper lobectomy. Ann Thorac Surg 2012;93:259-65. [PubMed]
- Regnard JF, Perrotin C, Giovannetti R, et al. Resection for tumors with carinal involvement: technical aspects, results, and prognostic factors. Ann Thorac Surg 2005;80:1841-6. [PubMed]
- Kim CW, Godelman A, Jain VR, et al. Postlobectomy chest radiographic changes: a quantitative analysis. Can Assoc Radiol J 2011;62:280-7. [PubMed]
- Nonaka M, Kadokura M, Yamamoto S, et al. Analysis of the anatomic changes in the thoracic cage after a lung resection using magnetic resonance imaging. Surg Today 2000;30:879-85. [PubMed]


