Chinese expert consensus on clinical use of non-invasive airway inflammation assessment in bronchial asthma
Introduction
The most essential pathological change of bronchial asthma (hereinafter referred to as asthma) is chronic airway inflammation. The severity of airway inflammation is closely associated with airway hyperresponsiveness (AHR), which is the origin of the clinical symptoms (1). Therefore, the assessment of airway inflammation, particularly by noninvasive methods, is of great significance to understand the pathophysiological characteristics of asthma, to guide the management and to achieve better overall control of asthma (2).
The Chinese Society of Chest Physicians and the China Asthma Alliance have convened a panel of experts in related fields to discuss and develop this consensus, referring to relevant international guidelines, and important literatures published within and outside China in recent years (3,4). This consensus aims to guide non-invasive airway inflammation assessment, especially the fractional exhaled nitric oxide (FeNO) measurement, including the methodology, quality control and results interpretation, in order to achieve better outcomes of clinical practice. In light of many aspects on the clinical significance and value of FeNO measurement remaining to be explored, this consensus also deals with current problems and proposes further research directions. Clinical studies, especially those with multi-center collaborations, are in dire need to improve the clinical practices and research in this field.
The relationship among airway inflammation, airway responsiveness and asthma control
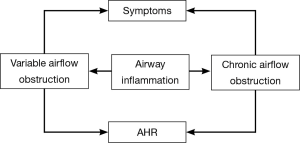
Asthma is a chronic inflammatory disease of the airways, involving various inflammatory cells, structural cells, and cellular mediators. The chronic inflammation leads to development and progression of AHR, widespread but variable and reversible airflow obstruction. The clinical manifestations include recurrent episodes of wheezing, breathlessness, chest tightness, coughing and other symptoms which frequently occur at night and/or early in the morning, and are often reversible either spontaneously or with treatment. Eosinophils (EOS) are the dominant inflammatory cells in the asthmatic airways. Increased number of EOS in the airways is the main pathological characteristic of asthma. EOS-released toxic proteins [e.g., major basic protein, eosinophil cationic protein (ECP)], inflammatory mediators (e.g., platelet activating factors, leukotrienes C4, D4), cytokines (e.g., IL-5), oxygen free radicals (e.g., superoxide anions, hydrogen peroxide and singlet oxygen) play essential roles in the pathogenesis of asthma.
AHR refers to the unduly strong and long-lasting contractile response of trachea and bronchi to endogenous and exogenous (including a variety of physical, chemical and immunological) stimuli. Over 99% of asthma patients present with varying severity of AHR, which is vital pathophysiological feature of this disorder. The airway response to stimuli in asthma patients can be 100-1,000 times higher compared with normal individuals. Therefore, airway responsiveness is not only an important measure for diagnosing or excluding asthma, but also for assessing asthma severity and treatment efficacy. Numerous studies have demonstrated that, controlling chronic airway inflammation is solely the effective approach to alleviate AHR, achieve and maintain asthma control, avoid acute exacerbations, improve pulmonary function and quality of life, reduce emergency visits and hospitalization, and ultimately cut down the mortality.
Thus, objective assessment of the airway inflammation makes a great sense for the diagnosis of asthma, selection and adjustment of treatment strategy, and achievement of asthma control.
The relationship among airway inflammation, AHR and asthma symptoms is illustrated in Figure 1.
The assessment methods and evaluation of airway inflammation
The assessment of airway responsiveness (5-7)
Airway responsiveness can be measured by bronchial provocation test (BPT). First introduced in 1873, BPT is used to induce airway constriction by inhalation of antigens or non-specific irritants. Since 1980s, this technique has been widely recognized and its methodology has been gradually refined to become more and more standardized. International and Chinese respiratory societies have issued guidelines on airway responsiveness measurement for better use of this technique in research and management of asthma. Depending on various irritants in use, airway responsiveness measurement is categorized as nonspecific and specific approaches. The non-specific approach involves irritants including histamine, methacholine, propranolol, distilled water, dry cold air, mannitol, leukotriene E4, adenosine monophosphate and ozone, among which, histamine and methacholine are commonly used. Specific measurement refers to the inhalation of liquid allergen extracts as the irritant, aiming at clarifying the relationship between AHR and specific allergens (8).
Methods
BPTs include direct and indirect provocation tests. The most preferred irritants used in direct provocation tests are histamine phosphate and methacholine. The test begins with measurement of baseline pulmonary function, followed by the provocation only if the baseline FEV1 is greater than 70% predicted. The dosage of the inhaled irritant escalates from the lowest concentration until the test becomes positive or asthmatic symptoms appear. Inhaled bronchodilator is then given to render pulmonary function back to or near baseline. If the test remains negative with maximum concentration of the irritant, the irritant inhalation should be discontinued and inhaled bronchodilators should be given.
Chiefly, two methods are available for the testing: (I) Using FEV1 as the indicator, such as Chai protocol (inhalation through five breathes of a fixed duration), Yan protocol (inhalation with a hand bulb nebulizer), and Cockcroft protocol (inhalation during tidal breathing). First, the subjects are determined for baseline FEV1 with forced expiration, and then they inhale histamine or methacholine in stepwise incremental concentrations. FEV1 is measured at each dose. When FEV1 drops by ≥20% from baseline or, physical examination reveals wheezing, or the maximum dose has been reached, the subjects should be given inhaled bronchodilator (salbutamol). Then the computer automatically calculates the PD20 to show a positive or negative BPT, as well as the degree of airway responsiveness. (II) Using forced oscillation technique for consecutive tracings of respiratory resistance, e.g., Astograph protocol. Subjects breathe calmly and inhale normal saline for recording of baseline resistance value. Thereafter, the subjects inhale stepwise incremental concentrations of methacholine while the respiratory resistance is continuously monitored. The entire nebulization system comprises of 12 serially arranged nebulizers. The first one contains normal saline and the last one contains bronchodilators. Nebulizers No. 2 to No. 11 deliver 2-fold incremental concentrations of methacholine (49 to 25,000 mg/L). The subjects inhale each dose of the irritant for 1 min. While the subjects inhale normal saline, the resistance is recorded as baseline resistance. Then, the device continuously records changes in respiratory resistance while the subjects continuously inhale the two-fold incremental concentrations of methacholine. When the respiratory resistance reaches 2 times the baseline level or, physical examination reveals wheezing, or the maximal dose has been reached, the subjects should be given inhaled bronchodilator (salbutamol). Finally, the computer automatically calculates Dmin and PD35 to show a positive or negative BPT, as well as the degree of airway responsiveness (9).
Evaluation indicators
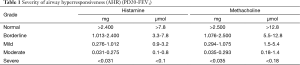
(I) Qualitative analysis: (i) If FEV1 or peak expiratory flow (PEF) drops ≥20% from the baseline values, specific airway conductance (sGaw) drops ≥35%, or wheezing occurs, the test can be evaluated as positive. (ii) If FEV1 drops by 15.9% to 20% after the inhalation of the maximum concentration of irritants with no shortness of breath or wheezing, the test is evaluated as probably positive. (iii) If the indicators do not reach the positive criteria above after the inhalation of the maximum concentration irritant, the result is evaluated as negative. (II) Quantitative analysis: PD20 refers to the cumulative dose of inhaled irritant when FEV1 drops by 20% from its baseline value. PC20 refers to the concentration when FEV1 drops by 20% from the baseline value. AHR is quantitatively graded by PD20 or PC20. For instance, the severity grading of AHR based on PC20-FEV1 (methacholine) is described as: <1.0 g/L, moderate to severe AHR; 1.0-4.0 g/L, mild AHR; 4.0-16 g/L, borderline AHR; >16 g/L, normal airway responsiveness (Table 1).
Full table
Clinical significance
(I) To help the diagnosis of asthma: positive airway responsiveness indicates a significant probability of asthma. If the positive result is accompanied with asthmatic symptoms, a diagnosis of asthma can be determined for most of the times. (II) To assess the severity and prognosis of asthma: the grade of AHR is positively correlated with the severity of asthma. Greater AHR indicates more severe asthma and a worse prognosis. (III) To guide the treatment of asthma and assess the efficacy. The higher airway responsiveness, the more necessity for active anti-inflammatory treatment is needed. If AHR is reduced after anti-inflammatory treatment in asthma patients, which indicates airway inflammation is controlled, the treatment can be stepped down. If airway responsiveness does not decrease after treatment, the patients probably need step-up treatment. (IV) To study the pathogenesis of asthma: since AHR is characteristic of asthma, investigating the mechanisms of AHR helps to understand the pathogenesis of asthma and contributes to the treatment of this disorder. (V) To indicate the airway responsiveness in other diseases associated with AHR. (VI) To clarify the causes (or allergens) of asthma by specific BPT.
Important tips
(I) The patient should be clinically stable, without asthma attack for at least 1 week. There is no obvious apnea or wheezing during the test. (II) The test is started by conducting a baseline spirometry to record the initial FEV1. For eligibility of BPT, FEV1 should mandatorily be ≥70% predicted value. (III) Patients with severe cardiopulmonary dysfunctions or hypertension, or women in pregnancy, are not eligible for this test. (IV) There might be a certain degree of risks during the BPT and rescue therapy should be available. The concentration of the inhaled drug should start from low dose, and increase gradually. Rescue agents include short acting β2 agonists, epinephrine, dexamethasone, oxygen and tracheal intubation equipment. An experienced clinician should be present during the test. The patient should be observed until FEV1 is back to or near baseline.
The measurement of airway responsiveness can indirectly reflect the severity of airway inflammation, and evaluate the efficacy of treatment and the prognosis of asthma. However, it demands sophisticated devices, and is also complex and time-consuming. BPT is not suitable for patients with poor pulmonary function (FEV1 <70% predicted value) and those with asthma exacerbations. Moreover, BPT is rather sensitive more than specific, and therefore a positive test can be seen in a variety of non-asthmatic conditions, like allergic rhinitis, cystic fibrosis, and viral infections. Finally, BPT may lag behind development of airway inflammation and may also trigger the exacerbation of asthma.
Induced sputum cytology
Sputum induction is a non-invasive procedure to induce sputum specimen by inhalation of hypertonic saline administrated by nebulisation in order to study the features and extent of airway inflammation by analyzing the cellular components and the soluble mediators. In addition to its value in understanding the mechanisms of airway inflammation in asthma, determining and differentiating diagnosis of many other respiratory disorders, induced sputum can be also of great value in investigating the treatment efficacy of drug and how they work. Induced sputum sample is easy to obtain. The sample can reflect the concentration of airway secretions in the natural (undiluted) state. Relevant inflammatory cells, mediators and cytokines can be directly detected. This can serve as a relatively direct reflection of airway inflammation and provide the quantitative information of airway inflammation, similar to those from BALF. The sensitivity and specificity of induced sputum cytology are superior to those of peripheral blood test. The procedure is non-invasive, repeatable and safe (10).
Test method
(I) Measure FEV1 at baseline. (II) The patient inhales salbutamol 200 µg/time for 2 times. (III) Re-measure FEV1 after 20 min. If FEV1 is >60% predicted, the following procedures can be performed. (IV) Instruct the patient to clear up nasal secretions, breathe calmly and inhale 3% hypertonic saline by an ultrasonic nebulizer for about 15 min. At 5, 10, 15 and 20 min of the induction, respectively, instruct the patient to gargle vigorously, cough forcefully and expectorate into a sputum collection container. (V) After each sputum induction, re-measure FEV1. If FEV1 declines by less than 10%, the test can be continued. If FEV1 decreases by 10% to 20%, the subjects should inhale salbutamol 200 µg again. If FEV1 did not improve after the inhalation of salbutamol, the test should be terminated. Otherwise, sputum induction can be continued. (VI) If the amount of induced sputum is not satisfactory, the above steps can be repeated with 4% and 5% hypertonic saline sequentially. Inhalation of sodium chloride solution in each concentration should be at least for 7 min. Cumulative nebulization duration should be no more than 30 min. (VII) End the induction, if the collected volume of sputum specimen is sufficient for the test (sputum volume should be ≥0.7 mL or 0.3 g, and percentage of mouth epithelial cells should be <5%), or the total inhalation duration reaches 30 min. (VIII) Discontinue the induction, if the patients present with chest tightness, cough, wheezing, and dyspnea, or if FEV1 decreases by over 20% from the baseline value, and FEV1 is <60% predicted. The subjects should be given inhaled salbutamol 200-400 µg until the symptoms are relieved.
Improved induction method: start by inhaling neubilized normal saline for 1 min, followed by 3%, 4%, and 5% of hypertonic saline for 1-2 min; collect the sputum specimens in accordance with the method described above; measure FEV1 before each procedure. If FEV1 declines by over 10%, or sufficient volume of sputum for a test has been collected, stop the procedure.
Evaluation indicators
Theoretically, the levels of all cells and mediators involved in airway inflammation, like EOS, neutrophils, ECP, NO3-/NO2-, and cytokines can be detected in induced sputum. Currently, induced sputum is mainly used for cell count in clinical practice and to determine the type of airway inflammation. The normal percentage of induced sputum EOS is <3.0% according to the international criteria (11), and it is <2.5% in Chinese cough guidelines (12). If the EOS count in induced sputum is above the normal range, the existence of eosinophilic airway inflammation can be determined.
Clinical significance
EOS in induced sputum, as one of the markers of airway inflammation, can timely reflect the level of airway inflammation, and sensitively indicate the responsiveness to treatment of glucocorticosteroids (13). In addition, a substantial proportion of patients present mixed or non-eosinophilic asthma. It is also of some clinical significance to observe the changes of other cell counts in the induced sputum.
Clinical application: (I) To differentiate asthma from other respiratory diseases. (II) To identify the types of airway inflammation in asthma and other respiratory diseases. (III) To study the causes for the exacerbation of airway inflammatory diseases. (IV) To predict the effect of glucocorticosteroids and guide medication options. Increased EOS in asthmatic sputum usually indicates that the patients can benefit more from glucocorticosteroids. (V) To evaluate the drug actions on airway inflammation. It is helpful to note the changes of cellular components and inflammatory mediators in induced sputum before and after the treatment for mechanistic study of drugs.
Important tips
(I) The inhalation of hypertonic saline is a non-specific stimulation on the airway. This can induce airway smooth muscle contraction and reduce the ventilation function, particularly in asthmatic patients. Therefore, the indications and contraindications of induced sputum test must be strictly followed. Lung function or PEF must be measured before the procedure. Sputum induction should not be considered for subjects who are not accessible to a pulmonary function test. (II) During the process of sputum induction, the operator must closely observe the response of the patients. Necessary rescue facilities should be provided at the site. (III) Inhaled β2 receptor agonist should be given before and after the induction. (IV) The sputum samples induced by the inhalation of hypertonic saline should be processed for the test as soon as possible. Because the “sputum” may contain saliva, the collected sample should be screened for quality. First, we need to identify whether the specimen is obtained from the upper respiratory tract or lower respiratory tract, which can be judged under a microscope by the mucus content and squamous cell counts. Few squamous cells are observed in the sputum from the subglottic airway. (V) The sticky part of the induced sputum or the sputum without squamous cell observed via an inverted microscope should be selected for examination. The total cell counts and cell viability, EOS and supernatant ECP levels in selected sputum specimens are higher than those in the unselected specimens. Probable dilution of induced sputum by saliva before the analysis may interfere with the results of tests. Another approach is to examine all the spit-ups including saliva and sputum. In comparison of these two methods, the latter may lead to a higher proportion of neutrophils in the classification count of non-squamous cells and a higher concentration of supernatant ECP. Whichever method being used, addition of 0.1% dithiothreitol (DTT) is needed to homogenize the specimen. DTT breaks up the disulfide bonds crosslinking the glycoprotein filaments and thereby dissolves the mucus and disperses the cells.
It is generally safe to carry out induced sputum examination in strict accordance with the procedures. The main complication is asthma episodes induced by bronchial spasm. Therefore, standard operating procedure (SOP) should be developed and appropriate rescue facilities should be configured to manage adverse reactions.
Absolute contraindications: (I) Any patients with FEV1 <1 L. (II) Recent massive hemoptysis. (III) Moderate to severe acute exacerbation, acute or chronic respiratory failure, pneumothorax or mediastinal emphysema, massive pleural or pericardial effusion, and severe heart failure. Relative contraindication: active tuberculosis.
Although induced sputum examination is simple to perform, it is time-consuming and work-consuming. The lack of standardized procedures and poor quality control of the operation may hamper the accuracy of the results and limit the widespread application of this technology in clinical practice.
Fractional exhaled nitric oxide (FeNO) measurement
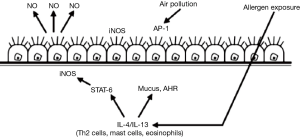
Nitric oxide, a gas molecule, is attracting widespread attention for its presence in the airways. Nitric oxide can be produced by various structural cells and inflammatory cells on the surface of airways under oxidation of nitric oxide synthase (Figure 2). The physiological roles of nitric oxide in the airways include neurotransmission, the dilation of blood vessels, the relaxation of airways and the regulation of inflammatory mediators. Although the roles of nitric oxide in asthmatic airways are not completely clear, a large number of studies have demonstrated that FeNO increases in asthma and declines after glucocorticosteroids therapy. In 1997, European Respiratory Society (ERS) developed the recommendations for FeNO measurement for the first time (14). The recommendations were later jointly revised by the American Thoracic Society (ATS) and ERS in 2005 (15). In 2011, ATS issued the guidelines for the clinical application of FeNO (3). In 2014, British National Institute for Health and Care Excellence (NICE) also promulgated relevant guideline documents online (16).
Allergens and other stimulating factors induce airway inflammation, with the release of cytokines by EOS and other cells, which results in the activation of nitric oxide synthase and the increase of nitric oxide levels in the airways.
Methods
Currently, the electrochemical detection method is recommended. Subjects generally take seats, rest for 5 min, and then try the best to exhale the air. Then, keep the mouthpieces (including filter/bacteria-filtering mouthpiece) of the nitric oxide analyzer tight in mouths. Inspire to reach the total lung capacity (TLC). Then, steadily and slowly expire at a constant flow rate of 50 mL/s (±5%) for 10 s (at least 4 s for children under 12 years of age, at least 6 s for adults and children over 12 years of age). The results are presented in ppb units.
Evaluation indicators
For American children and adults, the normal ranges of FeNO are 5-20 and 5-25 ppb, respectively; and the recommended cutoff values are 20 and 25 ppb, respectively (3,17). A multi-center clinical study in China showed the reference values of FeNO for healthy children (<15 years) and adults were 5-24 and 5-30 ppb, respectively, and suggested the cutoff values should be 24 and 30 ppb, respectively.
Clinical significance
(I) FeNO measurement is recommended to (i) assist the diagnosis and differential diagnosis of asthma; (ii) differentiate airway inflammation types and evaluate the severity; (iii) predict the responsiveness to inhaled corticosteroid (ICS) therapy; (iv) evaluate the adherence to ICS treatment; (v) assess the level of asthma control and predict the acute exacerbations; (vi) guide the adjustment of asthma treatment regimen (3). (II) FeNO is related to eosinophilic airway inflammation and of high diagnostic value for eosinophilic asthma rather than for non-eosinophilic asthma. As far as this is concerned, the diagnosis should be made cautiously. Recent study showed that in elderly patients with clinically stable asthma, the level of FeNO is not high and is not related to sample-size of study population, complications, treatments, symptoms and spirometry of subjects, suggesting that routine FeNO test is clinically relevant in elderly asthmatic patients (18). Few studies on cutoff values of FeNO have been reported in China. Further epidemiological data and more rigorously designed randomized controlled clinical studies are needed.
Important tips
(I) FeNO should be measured in the room. The indoor air concentration of nitric oxide can affect the test results (nitric oxide in ambient air is required <5 ppb). Before the test, subjects should have been kept on abstinence of alcohol for 4 h, of nitrate-containing food for 2 h, of water and other food for 1 h. Smoking and strenuous exercise are also prohibited for 1 h before the test. Inspiration and expiration during the test should be continuous. Do not hold and pause breath (14-16). (II) The following factors can up-regulate FeNO expression: food and medicines containing nitrates, L-arginine, inhaled β-agonists, atopy and viral infections. The following factors can down-regulate FeNO expression: smoking, drinking, inhaled or oral corticosteroids, leukotriene receptor antagonists, pulmonary function test, induced sputum test, BPT and omalizumab. As for the effects of gender, pregnancy, menstrual period and caffeine on FeNO, the current results are controversial (16-19).
In conclusion, FeNO is a novel, non-invasive and easy-to-use biomarker that relates prominently to allergic airway inflammation. FeNO test may contribute to the typing of airway inflammation, diagnosis, evaluation and treatment of asthma. Currently, FeNO test has been wide-spreading in clinical practice.
Exhaled breath condensate (EBC) analysis
EBC analysis is a new method to evaluate the inflammation and oxidative stress of airways by measuring the biochemical markers in EBC.
Measurement methods
Patients directly exhale the air into a collection tube. Then the tube is immersed in an ice bath or other condensing apparatus (below 4 °C). As the temperature decreases, the exhaled gas condenses into EBC. It usually takes 10-15 min to collect 2-4 mL of EBC. Special tests are always needed due to the generally low concentration of liquid medium in EBC.
Evaluation indicators
Nowadays, commonly recognized EBC biomarkers include hydrogen peroxide (H2O2), 8-iso-prostaglandin, products of nitric oxide, leukotrienes and pH value. Few studies focus on other EBC indicators such as prostaglandin, glutathione, aldehydes, cytokines, adenosine, endothelin 1, interferon α, cations, macrophage-related media and growth factors. The conclusions are also controversial. (I) H2O2: H2O2 is an important product of the oxidation cascade, and is usually measured with spectrophotometry or fluorescence spectrometry. Studies have showed that children with asthma have significantly increased H2O2 levels (20). A meta-analysis reported that the H2O2 level in non-smoking asthma patients is negatively correlated with FEV1, and dramatically declines after glucocorticosteroids therapy (21). (II) 8-iso-prostaglandin: this is a prostaglandin-related analogue compound that can be measured by enzyme immunoassay (EIA), gas chromatography/mass spectrometry (GC/MS) or liquid chromatography/mass spectrometry (LC/MS). A majority of studies showed that the level of 8-iso-prostaglandin was elevated in asthmatic children (20,22-27), and correlated with the severity of asthma (22). (III) Products of nitric oxide: 3-nitro-tyrosine and nitrogen oxides (NOX) produced by nitric oxide pathway can be detected in EBC and measured by EIA. Several studies showed that the level of nitrite and the ratio of nitrite/nitrate significantly increased in children with asthma (28,29). (IV) Leukotrienes: leukotriene pathway is involved in the asthmatic airway inflammation. Leukotrienes can be detected by EIA, GS/MS or LC/MS. Most studies reported significantly higher levels of leukotrienes in children with asthma (23,25-27,30,31). (V) pH: the pH value of EBC can be measured with a pH meter or blood gas analysis. It is significantly low in patients with asthma, and even lower in severe cases (32,33).
Clinical significance
Literatures (meta-analysis) reported the correlation between the levels of EBC indicators and asthma control. The severity of asthma is related to the levels of 8-iso-prostaglandin, nitrite and nitrate. The level of asthma control is related to the level of 8-iso-prostaglandin, interferon γ and IL-4 (21). Further studies are needed to determine whether EBC biomarkers can be used as independent predictors for evaluating the onset and control of asthma (34).
The biomarkers in EBC, which can reflect the severity of airway inflammation, have been widely used for asthma research in recent years. Unfortunately, the very low and unstable level of mediators in EBC render sophisticated instruments needed for the detection, and therefore deters the widespread use of EBC test in clinical practice.
Other assessments
Since various inflammatory cells, cell components and inflammatory mediators interact in airway inflammation, novel indicators for evaluation have attracted increasing interest in recent years.
EOS count in peripheral blood is a reliable indicator of eosinophilic asthma phenotype (35). Increased EOS indicates acute exacerbation of eosinophilic asthma. In addition, peripheral EOS count can be used to monitor the effects of asthma medication and immune intervention. For example, peripheral EOS count >150/µL suggests that the asthma patient is suitable for mepolizumab and other anti-IL-5 therapy (36). Moreover, a significant increase in the daily variation of peripheral EOS count can also indicate an episode of asthma (37).
Urinary leukotriene E4 (ULTE4) can be significantly increased in acute exacerbation of childhood asthma. The concentration of ULTE4 may reflect the severity of airflow obstruction in asthmatic children and predict the risk of asthma onsets triggered by second-hand smoking and air pollution (38). Meanwhile, ULTE4 level or ULTE4/FeNO ratio may be used to predict the response to leukotriene receptor antagonists in children with mild to moderate asthma (39).
The serum levels of periostin, an extracellular matrix protein, may be used to determine whether the patients have Th2-driven airway inflammation and to screen suitable candidates for anti-IL-13 therapy (40).
Bromotyrosine (BrY) is a highly specific indicator for EOS activation. Urinary BrY level quickly increases during the acute exacerbation of asthma (41).
S-nitrosoglutathione (GSNO) is an important bioactive factor against AHR in vivo. FeNO decreases rapidly in asthma patients after the inhalation of GSNO. FeNO decrement rate can be detected with GSNO provocation test. This can be used to identify individuals with high EOS counts and high concentration of GSNO reductase (42).
The level of urinary F2-isoprostanes (F2IsoPs) significantly increases in asthma patients and it is closely related to the severity of asthma (43).
The increase in serum arginase is closely related to the acute exacerbation and poor control of asthma (44). The arginase activity is inversely associated with FeNO levels in patients with severe asthma (45). Serum arginase level and its activity may both be important indicators of airway inflammation, particularly for patients with glucocorticosteroid-resistant asthma.
Clinical significance of FeNO measurement
Diagnosis and differential diagnosis of asthma
The diagnosis of typical asthma is based on the symptoms and the medical history. For individuals with atypical symptoms and needs for differential diagnosis, laboratory tests should be carried out. Currently, the most commonly used tests in clinical practice include BPT, bronchodilation test and PEF monitoring. The above tests mainly reflect ventilation function but could not confirm on the existence of airway inflammation. A large number of clinical studies have been conducted on the diagnostic value of FeNO, but with mixed conclusions. In several studies from the western hemisphere, where clinical symptoms and positive BPT or bronchodilator test were set as the gold standard for the diagnosis of asthma, the diagnostic cutoff values varied among investigators (7, 12, 16 and 20 ppb). Overall, those results linked the diagnostic performance of FeNO measurement for asthma to a sensitivity ranging from 32% to 88%, a specificity from 79% to 93%, a positive predictive value from 70% to 80%, and a negative predictive value from 61% to 92% (44-47). A Japanese study reported that allergic rhinitis and smoking can significantly interfere with the measurement results. Therefore, the optimal diagnostic cutoffs for smokers and individuals with rhinitis should be adjusted to 18 and 28 ppb (48). A clinical study in China demonstrated that with symptoms and positive BPT or bronchodilator test as the gold standard for the diagnosis of asthma, and FeNO ≥36.5 ppb as the cutoff value, the diagnosis of asthma with FeNO measurement was linked to a sensitivity of 79.2%, a specificity of 94.3%, a positive predictive value of 92.7%, a negative predictive value of 83.3%, and an accuracy rate of 87.1% (49). Another Chinese study on 358 cases of childhood asthma reported a favorable negative predictive value of FeNO when 17.9 ppb was set as the cutoff value; however, the positive predictive value was only 56.33% (50).
For individuals with atypical asthmatic symptoms, it is an important diagnostic method to determine AHR by BPT. Negative BPT results usually help to exclude the diagnosis of asthma. However, not all individuals can complete BPT in clinical practice, such as children who are unable to cooperate for a pulmonary function test and patients who have ventilatory dysfunction. Relevant clinical studies reported conflicting results on whether FeNO could be used as an alternative marker for AHR. In 2005, Berkman et al. reported that the diagnostic value of FeNO was equivalent to that of BPT when the cutoff value was 7 ppb (47). However, other studies showed the extent of correlation between FeNO and AHR were not consistent, suggesting that the relationship between nitric oxide metabolism and AHR may be very complex (51).
Although the results of the above studies showed certain sensitivity and specificity of FeNO for the diagnosis of asthma, current guidelines have not recommended use of FeNO alone for asthma diagnosis (3), based on following considerations: (I) The FeNO levels in asthma patients overlap with the ranges in healthy individuals. Thus, it is difficult to set a normal predicted value of FeNO. The value of the reference range obtained from healthy populations should be inferior to that obtained from the patients. Therefore, ATS guidelines recommend using cutoff values, rather than the reference ranges, for the interpretation of FeNO. (II) The airway inflammation in asthma may include different phenotypes, such as eosinophilic phenotype, neutrophilic phenotype, mixed cellular phenotype and paucigranulocytic asthma phenotype. Eosinophilic phenotype accounts for approximately 50-60% of asthma cases. FeNO mainly reflects the eosinophilic airway inflammation. For other phenotypes of airway inflammation or for patients who already received glucocorticosteroid therapy, FeNO test results may be negative, which is a major setback for FeNO in the diagnosis of asthma. (III) The diagnostic cutoff values in use differed across studies. Some of them which focused on determining the optimal cutoff values of FeNO measurement in diagnosing asthma, the results varied from 20 to 26 ppb (52,53). While the higher cutoff value corresponds to higher specificity and lower sensitivity of the asthma diagnosis, and vice versa, the best cutoff value for diagnosing asthma has not been recognized yet. (IV) Genetics, age, gender, atopic status, body weight, height, current smoking status and diet can interfere with FeNO levels (54). In some patients with chronic obstructive pulmonary disease (COPD) or eosinophilic bronchitis, FeNO levels may also increase, which might lead to false-positive results in the diagnosis. (V) The levels of FeNO may suggest a likelihood of having asthma, but are not sufficiently robust to confirm or exclude asthma. The diagnostic values of high versus low levels of FeNO are interpreted in different scenarios. For patients with typical symptoms, increased FeNO level is supportive of the diagnosis of asthma; for patients with atypical symptoms, a low FeNO level is valuable for excluding asthma. Meanwhile, other diseases that may cause similar symptoms need to be differentiated, such as reactive airway dysfunction syndrome, COPD, bronchiectasis, cystic fibrosis, primary ciliary dyskinesia, bronchial hyperresponsiveness syndrome after long-term viral infection, vocal cord dysfunction, anxiety-hyperventilation, gastroesophageal reflux disease, heart disease and pulmonary hypertension.
As mentioned above, in view of various confounding factors in FeNO test and the overlap between healthy subject and asthma patients, we conclude that: (I) Current evidence is insufficient to recommend FeNO as a routine diagnostic tool in clinical practice. (II) FeNO detection is still of high diagnostic value for individuals with certain clinical characteristics. For early onset and atopic individuals, high level of FeNO strongly suggests the diagnosis of asthma. (III) The diagnostic significance of negative predictive value of FeNO is superior to that of the positive predictive value. The lower FeNO levels can be more robust for excluding the diagnosis of asthma, in particular, an extremely low level of FeNO may suggest the least possibility of asthma. However, clinical manifestations and other laboratory investigations are still needed for a comprehensive evaluation. (IV) A complete diagnosis of asthma includes not only the identification of asthma, but also the evaluation of the severity and control of asthma. In the future, the clinical and inflammatory phenotypes might also be included in asthma diagnosis. Therefore, from a broader perspective, FeNO test will enrich the diagnostic tools of asthma. Especially in identifying the phenotypes of airway inflammation for further prediction of glucocorticoid responsiveness, FeNO test may be advantageous, which will be discussed in details in the text below.
Differentiation of airway inflammation phenotypes and assessment of airway inflammation
The main phenotypes of airway inflammation in asthma are described as eosinophilic, neutrophilic, mixed and paucigranulocytic. Different airway inflammation phenotypes are associated with different clinical features and treatment responses, and ultimately, different clinical outcomes. Identification of these phenotypes and assessment of the airway inflammation may guide clinicians in the selection and adjustment of treatment strategy.
FeNO mainly reflects eosinophilic inflammation of airways (3). Many studies showed that in glucocorticoid-naïve adults or children with mild to moderate asthma, FeNO is positively correlated to EOS counts in the sputum, BALF, lung tissue and peripheral blood (55-62). The levels of FeNO are significantly related to ECP in peripheral blood and BALF (55,63). In nonsmoking asthma patients, the sensitivity and specificity of FeNO (>36 ppb) are 78% and 72% respectively for the identification of EOS >3% in induced sputum (60). In determining the presence or absence of eosinophilic inflammation, the negative predictive value of low levels of FeNO is more significant (64,65). But for asthma patients with a history of smoking or glucocorticoid therapy, FeNO level is less valuable for the determination of eosinophilic airway inflammation (66). ATS guidelines [2011] (3) recommended FeNO as the marker of eosinophilic airway inflammation and classified FeNO levels into low level (<25 ppb in adults, <20 ppb in children), intermediate level (25-50 ppb in adults, 20-35 ppb in children) and high level (>50 ppb in adults, >35 ppb in children) (Table 2). By ATS recommendations, FeNO <25 ppb in adults (<20 ppb in children) can be used to initially exclude eosinophilic inflammation and glucocorticoid therapy is not recommended (strong recommendation, high level of evidence); FeNO >50 ppb in adults (>35 ppb in children) indicates eosinophilic inflammation and glucocorticoid therapy is recommended for symptomatic patients (strong recommendation, moderate quality of evidence); FeNO between 25 and 50 ppb in adults and (20-35 ppb in children) indicates eosinophilic inflammation but it should be interpreted in the clinical context (strong recommendation, low level of evidence).

Full table
FeNO levels can be used to identify patients with severe asthma and persistent eosinophilic airway inflammation. Studies suggest that compared with those in mild-moderate asthma group, patients in severe asthma group have higher FeNO levels (medians, 38 and 27 ppb) and sputum EOS counts (medians, 11.8% and 0.8%) (67). Even in patients with severe asthma being on ICS or oral steroid therapy, FeNO is still related with the eosinophilic inflammation in bronchial mucosa biopsies. FeNO cut point greater than 72.9 ppb demonstrates a sensitivity of 56% and a specificity of 100% for the identification of persistent eosinophilic airway inflammation (56,68).
Evaluation of the response to inhaled corticosteroid (ICS) therapy
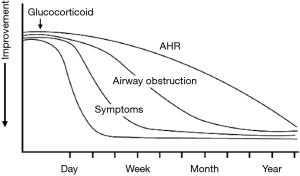
The response to ICS treatment is heterogeneous in patients with asthma. It is critical to select the optimal dose of ICS in individualized treatment to reduce adverse reactions, and meanwhile to maintain asthma control and reduce the risk of exacerbations. Given the correlation between the presence of eosinophilic inflammation and the response to corticosteroid therapy in airways disease (69), the monitoring of FeNO levels helps identify those who might benefit from corticosteroid therapy, and evaluate individual’s response to corticosteroids. The improvement in FeNO levels appears earlier compared with pulmonary function, bronchodilator responsiveness, PEF variability and AHR (70-72) (Figure 3). In steroid-naive asthmatic adults or children, FeNO levels and anti-inflammatory effects of ICS exhibit a significant dose-response relationship (73), and the decline in FeNO levels was accompanied by the improvement of inflammation, lung function, airway responsiveness and the level of asthma control (74,75). In steroid-naive asthma patients, FeNO cutoff point of 47 ppb or greater yields a high positive predictive value for ICS responsiveness; and a FeNO cutoff point within the range of 20-30 ppb yields a higher negative predictive value for the responsiveness to ICS therapy (76). Even when patients do not demonstrate EOS in the sputum, FeNO is still highly predictive of steroid response (at a cutoff point of 33 ppb) (77). On the other hand, FeNO monitoring contributes to screening asthma patients with “uncontrolled inflammation” who, however, have received corticosteroid (78). For patients with well-controlled asthma and FeNO level above 25 ppb (ACT score >20), continuous ICS therapy may reduce FeNO level, significantly improve FEF25-75 and other indicators of small airway function (79). ATS guidelines [2011] (3) suggest that high levels of FeNO usually indicate a significant response to ICS therapy. The criteria for steroid response (see Table 3) is a reduction of >20% in FeNO for values >50 ppb, or >10 ppb for values <50 ppb as the cut point (weak recommendation, low level of evidence). ATS guidelines point out that FeNO can provide important information for clinicians to decide who might benefit from steroid treatment. However, these outcomes may differ depending on the target population and epidemiology of eosinophilic inflammation.

Full table
The decline in FeNO levels is related to the duration of steroid therapy. A study showed that FeNO gradually decreased after regular inhalation of budesonide for 1 week, and significantly fell after 3-4 weeks (80).
FeNO is also significant to evaluate the response to treatment in patients with severe asthma. In patients with refractory asthma, the sensitivity, specificity, positive predictive value and negative predictive value of FeNO (≥30 ppb) to evaluate the response to high-dose ICS or systemic corticosteroid treatment were 87.5%, 90.6%, 87.5% and 90.6%, respectively (81).
Evaluation of patient compliance to inhaled corticosteroid (ICS) therapy
The patient compliance to ICS treatment is a prerequisite for good control of asthma. However, the overall compliance to ICS treatment is relatively low. The reasons may include: patient concerns about drug-related adverse reactions, under-estimation of the disease severity and inadequate patient-physician communications (4). A study with continuous monitoring of FeNO levels after ICS therapy in asthmatic children and synchronous assessment of their compliance to treatment showed that, unlike pulmonary function test, FeNO levels were significantly correlated with patient compliance (82); they usually increased with in subjects with poor compliance (83). The authors believe raising parents awareness of therapeutic agents and FeNO monitoring help improve medication compliance in children, so as to achieve better asthma control. In a study on the FeNO monitoring in patients with ICS refractory asthma, FeNO levels decreased significantly in those with good adherence. Therefore, under certain conditions, FeNO can also be used as an evaluation indicator for treatment compliance in patients with refractory asthma (84). After ICS treatment, FeNO level changes more rapidly than other inflammatory markers. Therefore, it is more pragmatic to be used for monitoring patient adherence and therapeutic response.
Assessment of asthma control and prediction of acute exacerbation
The levels of asthma control are classified as controlled, partially controlled and uncontrolled. The evaluation indicators consist of daytime/night-time symptoms, limitations of activities, the need for rescue medications, pulmonary function, and acute exacerbations (85). Asthmatic children with lower FeNO levels (<20 ppb) have better pulmonary function and asthma control, when compared with those with higher FeNO levels (≥20 ppb) (86). FeNO is correlated to TLC. The increase in FeNO levels is positively correlated with the elevation of TLC, which is related to the acute exacerbations and poor control of asthma (87). High levels of FeNO (>50 ppb for adults, >35 ppb for children) or elevation of FeNO levels by >40% from the levels at remission suggests uncontrolled asthma or aggravation of eosinophilic airway inflammation (88).
In summary, FeNO as a direct indicator of airway inflammation, in combination with clinical indicators, can comprehensively reflect the profile of asthma control. When FeNO is used to assess asthma control, clinical workers should be prompted for exclusion of other factors (such as sinusitis, anxiety, gastro-esophageal reflux, obesity or persistent allergen exposure) which can also affect the levels of FeNO. Anyway, these factors reflect certain limitations with FeNO measurement (3).
In children and adults with allergic asthma, FeNO levels above 300% predicted value may predict a greater need for SABA and increased risk of acute episodes (89) in the following year. The likelihood of acute exacerbation in the future is 85% in clinically stable asthma with FeNO ≥28 ppb and FEV1 ≤76%. However, the likelihood of acute exacerbation in the future is nearly zero in asthma patients with FeNO ≤28 ppb and FEV1 ≥76% predicted (90).
Guiding adjustment of treatment strategies
The management strategy of asthma recommended by GINA and relevant guidelines are mainly based on the clinical symptoms and pulmonary function instead of airway inflammation as the nature of asthma. Treatment strategy for asthma based on the detection of airway inflammation has been expected over a long time. In several previous studies, ICS dose was adjusted according to EOS count in induced sputum. The results proved this strategy was more effective to control asthma than that simply based on symptoms or pulmonary function (91). The level of FeNO is related to the eosinophilic inflammation of airways. Theoretically, the asthma treatment strategy based on FeNO should be an ideal solution. In recent years, dozens of relevant clinical studies on adults and children with asthma have been reported, wherein the dose of ICS was usually adjusted based on a combination of clinical assessment and FeNO level, stepping up or down when FeNO reached a certain cut point. Most of the studies showed treatment strategy based on FeNO can maintain favorable asthma control with the same or a lower dose of ICS (92), compared to the adjustment of therapeutic strategies simply based on clinical symptoms. In addition, some studies demonstrated the treatment strategy could help improve airway inflammation and reduce airway responsiveness (93). A meta-analysis of clinical trials on whether FeNO-based management can reduce the risk of acute episodes of asthma showed that the rates of acute exacerbation declined by 47% and 30% in adults and children, respectively, without increasing the ICS dose (94,95). In 2011, Powell et al. (96) completed a double-blind, randomized controlled study in which 242 pregnant women with asthma were managed based on FeNO. The treatment was up- or down-stepped according to asthma test score (ACQ), or a combination of ACQ and FeNO level (29 bbp as cut point). The results showed the rate of acute exacerbation was 25% in the FeNO group vs. 41% in the control group. Moreover, the ICS dosing was lower and neonatal hospital stay was significantly reduced in FeNO group. In view of the special significance of drug safety in pregnant women with asthma, the results have been widely noted and published in Lancet (96). However, some studies turned out negative results (97). In a study on childhood asthma, the patients had daily FeNO test at home using a portable FeNO monitor, while the dose of ICS was adjusted with telemonitors. There was no significant difference in improvement of asthma control between this approach and simply symptom-based treatment adjustment (98).
A recent systematic assessment of published randomized controlled clinical trials on FeNO-guided asthma treatment in the past years showed that the inconsistency among these results are mainly due to different methodologies (99) including disparities in FeNO cutoff values/cut points, patient compliance, LABA and other confounding factors. Therefore, it is necessary to apply a uniform adjustment value and more precise studies should be designed in order to further evaluate the values of asthma treatment strategies guided by traditional methods and biomarkers like FeNO. FeNO measurement is technically mature, easy to operate, and well reproducible; however, these advantages can be outweighed by the increased financial burden of patients if routine FeNO monitoring is proposed, given the current situation in China. In addition, routine therapeutic dose of ICS is safe for most asthma patients. Therefore, the adjustment of asthma treatment should still rely on strategies based on the clinical context, as recommended in GINA guidelines. As for those with potential safety concerns about ICS, such as childhood asthma, asthma in pregnancy and refractory asthma that requires large doses of glucocorticoids, FeNO-based treatment strategies contribute to more accurate adjustment and least adverse reactions of glucocorticoid load and other drugs, under the premise of maintaining asthma control. Future research should focus more specifically on the above asthma populations. For instance, in a recent study, 102 patients with suboptimal asthma control underwent stepwise incremental dosing of ICS therapy within one month, based on FeNO levels; for those whose asthma remained uncontrolled, oral corticosteroid therapy for another month was given. Finally, asthma control was achieved in 53 patients (52%) (81).
In addition, dynamic FeNO monitoring helps to decide on the time for tapering or withdrawal of ICS. A study on childhood asthma showed that high FeNO levels (>47 ppb) may predict failure of asthma control after tapering or withdrawal of ICS, with the sensitivity and specificity being 71% and 93%, respectively (100). Meanwhile, low FeNO levels (<22 ppb) can predict the successful tapering or withdrawal of ICS in 92.5% of the times (101).
Since the advent of FeNO testing, unique advantages of this technique have been shown in the diagnosis and differential diagnosis of asthma, determination of airway inflammation types, prediction and assessment of treatment response, assessment of asthma control and guidance of treatment adjustment. Along with the growing experience in clinical practice and accumulation in understandings, we look forward to a greater role of FeNO technology in the management of asthma. The use and significance of FeNO testing in asthma management are summarized in Table 4.

Full table
Future directions and prospects of research
Over the past decades, based on in-depth understanding of asthma as a special chronic airway inflammatory disorder, non-invasive assessment of airway inflammation has been widely recognized and used by respiratory physicians in China. A great deal of achievements has been obtained, and experience accumulated. However, the strength of research varies. There is still a lack of convincing data from large-sample clinical trials. Opinions about the non-invasive laboratory techniques for detection of airway inflammation, interpretations and clinical evaluation of test results remain disputed. Chinese experts in the relevant fields developed the present consensus on the commonly concerned issues in clinical practice, based on currently available evidence and their decades of clinical expertise. The Committee acknowledges that this consensus is just an interim statement based on the current body of evidence. The purpose of publishing this document is to call for attention of Chinese respiratory physicians to the detection of airway inflammation, to inspire clinical research in this field in China and promote the use of this technique in clinical practice.
BPT is an important tool for the diagnosis of asthma. AHR revealed by BPT is closely related to airway inflammation. However, airway inflammation is not the only mechanism underlying AHR, and thus the both are not to be mentioned exchangeably. BPT does not directly reflect the airway inflammation. Further studies are warranted to elucidate on the diagnostic value of BPT and its relationship with airway inflammation under special conditions, for example, the probable existence of BPT-negative airway inflammation. For airway responsiveness, large-sample clinical trials with longer follow-up duration are in dire need to determine its role in the pathogenesis and development of asthma, the regularities and significance of dynamic variation after treatment, the predictive value for the prognosis and risk of asthma.
Induced sputum cytology is a proven, simple method not only useful for counting of inflammatory cells, but also for detecting various components in the supernatant. It is of high value in the differentiation of airway inflammation types and the prediction of the ICS treatment response. Although induced sputum cytology has been currently conducted in many hospitals in China, a SOP for specimen collection, operation and smear examination has not yet been established in most laboratories, which greatly hampers the inter-laboratory recognition of test results in this country. Therefore, we appeal for establishment of the national SOP for this technique, training centers and quality control system so that induced sputum would become a routine examination in clinical practice in the near future.
Nearly 20 years after its first introduction, FeNO testing has hallmarked a major improvement in the field of asthma research. Recently, a surge of data further broadens the application of FeNO and heralds new challenges. The results of a multi-center study on the predictive value of FeNO in normal Chinese population have been released. However, the optimal cut points for the diagnosis and treatment adjustment in asthma patients are still in expectation. To this end, large-sample clinical trials need to be carried out to obtain more reliable data in different geographic regions, ethnic and age groups. Meanwhile, professional training of clinicians should be reinforced to enable them properly interpret the results of FeNO test based on clinical findings. We also expect miniaturized, budget-friendly detectors to be developed in the future to make possible the use of FeNO test in daily management of asthma. The stability, repeatability of non-velocity-dependent FeNO measurement technique, and whether it reflects the NO concentrations in segment air stream, are still need to be verified by rigorous clinical trials.
The technique to detect inflammatory cells and mediators in peripheral blood and body fluid is mature, and allows easy collection of specimens; however, the drawback is that the indicators can hardly reflect the real condition of airway inflammation timely. The findings of detection are less correlated to what is happening in the lungs. Therefore, it would be inappropriate to overestimate their roles in the evaluation of airway inflammation. Meanwhile, exploratory efforts for novel indicators with higher specificity and sensitivity seem indispensable.
Acknowledgements
The consensus is endorsed by a panel of experts (in alphabetical order): Ping Chen (The General Hospital of Shenyang Military), Lingfei Kong (The First Hospital of China Medical University), Ao Liu (The Kunming General Hospital of Chengdu Military), Rongyu Liu (First Affiliated Hospital of Anhui Medical University), Huahao Shen (The Second Affiliated Hospital of Zhejiang University School of Medicine), Huanying Wan (Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine), Changzheng Wang (Xinqiao Hospital, Third Military Medical University), Changgui Wu (Xijing Hospital, Fourth Military Medical University), Hua Xie (The General Hospital of Shenyang Military), Dong Yang (Zhongshan Hospital, Fudan University), Yadong Yuan (The Second Hospital of Hebei Medical University), Xin Zhou (First People’s Hospital, Shanghai Jiaotong University), Weihe Zhao (Ningbo No.2 Hospital).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Asthma Assembly of Chinese Thoracic Society. Chinese Consensus Statement on Bronchial Asthma Management. Chinese Journal of Internal Medicine 2013;52:440-3.
- Lin J. Achieving overall asthma control is the core of effective asthma management. Chinese Journal of Internal Medicine 2014;53:594-5. [PubMed]
- Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602-15. [PubMed]
- Bjermer L, Alving K, Diamanta Z, et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respiratory Medicine 2014;108:830-41. [PubMed]
- Lung Function Assembly of Chinese Thoracic Society. Guideline of lung function test (section 3)—histamine and methacholine bronchial provocation tests. Chinese Journal of Tuberculosis and Respiratory Diseases 2014;37:566-71.
- Zhang H, Wu YF, Huang JF, et al. Chinese Consensus Statement on Pediatric Lung Function Test and Assessment. The Journal of Clinical Pediatrics 2014;32:104-14.
- Zheng JP, Chen RC. Bronchial provocation test/lung function-basic and clinical medicine. Guangdong: Guangdong Technical Press; 2007:93-115.
- Chinese Thoracic Society. The measurement of airway responsiveness (bronchial provocation test). Chinese Journal of Tuberculosis and Respiratory Diseases 1997;20:265-7.
- Mochizuki H, Arukawa H, Tokuyama K, et al. Bronchial sensitivity and bronchial reactivity in children with cough variant asthma. Chest 2005;128:2427-34. [PubMed]
- Gibson PG. How to measure airway inflammation: induced sputum. Can Respir J 1998;5 Suppl A:22A-6A.
- Simpson JL, McElduff P, Gibson PG. Assessment and reproducibility of non-eosinophilic asthma using induced sputum. Respiration 2010;79:147-51. [PubMed]
- Asthma Assembly of Chinese Thoracic Society. Guideline for Cough Diagnosis and Treatment (2009 version). Chinese Journal of Tuberculosis and Respiratory Diseases 2009;32:407-13.
- Wark PA, Gibson PG, Fakes K. Induced sputum eosinophils in the assessment of asthma and chronic cough. Respirology 2000;5:51-57. [PubMed]
- Kharitonov S, Alving K, Barnes PJ. Exhaled and Hasal nitric oxide measurements: recommendations. The European Respiratory Society Task Force. Eur Respir J 1997;10:1683-93. [PubMed]
- American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912-30. [PubMed]
- National Institute for Health and Care Excellence. Measuring fractional exhaled nitric oxide concentration in asthma: NIOX MINO, NIOX VERO and NObreath. Available online: http://www.nice.org.uk/guidance/dg12
- Taylor DR, Pijnenburg MW, Smith AD, et al. Exhaled nitric oxide measurements: clinical application and interpretation. Thorax 2006;61:817-27. [PubMed]
- Columbo M, Wong B, Panettieri RA Jr, et al. Asthma in the elderly: the role of exhaled nitric oxide measurements. Respir Med 2013;107:785-7. [PubMed]
- Spitale N, Popat N, McIvor A. Update on exhaled nitric oxide in pulmonary disease. Expert Rev Respir Med 2012;6:105-15. [PubMed]
- Robroeks CM, van de Kant KD, Jöbsis Q, et al. Exhaled nitric oxide and biomarkers in exhaled breath condensate indicate the presence, severity and control of childhood asthma. Clin Exp Allergy 2007;37:1303-11. [PubMed]
- Teng Y, Sun P, Zhang J, et al. Hydrogen peroxide in exhaled breath condensate in patients with asthma: a promising biomarker? Chest 2011;140:108-16. [PubMed]
- Baraldi E, Ghiro L, Piovan V, et al. Increased exhaled 8-isoprostane in childhood asthma. Chest 2003;124:25-31. [PubMed]
- Kiełbasa B, Moeller A, Sanak M, et al. Eicosanoids in exhaled breath condensates in the assessment of childhood asthma. Pediatr Allergy Immunol 2008;19:660-9. [PubMed]
- Shahid SK, Kharitonov SA, Wilson NM, et al. Exhaled 8-isoprostane in childhood asthma. Respir Res 2005;6:79. [PubMed]
- Baraldi E, Carraro S, Alinovi R, et al. Cysteinyl leukotrienes and 8-isoprostane in exhaled breath condensate of children with asthma exacerbations. Thorax 2003;58:505-9. [PubMed]
- Zanconato S, Carraro S, Corradi M, et al. Leukotrienes and 8-isoprostane in exhaled breath condensate of children with stable and unstable asthma. J Allergy Clin Immunol 2004;113:257-63. [PubMed]
- Mondino C, Ciabattoni G, Koch P, et al. Effects of inhaled corticosteroids on exhaled leukotrienes and prostanoids in asthmatic children. J Allergy Clin Immunol 2004;114:761-7. [PubMed]
- Ratnawati Morton J. Exhaled breath condensate nitrite/nitrate and pH in relation to pediatric asthma control and exhaled nitric oxide. Pediatr Pulmonol 2006;41:929-36. [PubMed]
- Carpagnano GE, Barnes PJ, Francis J, et al. Breath condensate pH in children with cystic fibrosis and asthma: a new noninvasive marker of airway inflammation? Chest 2004;125:2005-10. [PubMed]
- Steiss JO, Rudloff S, Landmann E, et al. Effect of inhaled corticosteroid treatment on exhaled breath condensate leukotriene E(4) in children with mild asthma. Allergy Asthma Proc 2008;29:371-5. [PubMed]
- Lex C, Zacharasiewicz A, Payne DNR, et al. Exhaled breath condensate cysteinyl leukotrienes and airway remodeling in childhood asthma: a pilot study. Respir Res 2006;7:63. [PubMed]
- Nicolaou NC, Lowe LA, Murray CS, et al. Exhaled breath condensate pH and childhood asthma: unselected birth cohort study. Am J Respir Crit Care Med 2006;174:254-9. [PubMed]
- Vlasić Z, Dodig S, Cepelak I, et al. Iron and ferritin concentrations in exhaled breath condensate of children with asthma. J Asthma 2009;46:81-5. [PubMed]
- Thomas PS, Lowe AJ, Samarasinghe P, et al. Exhaled breath condensate in pediatric asthma: promising new advance or pouring cold water on a lot of hot air? A systematic review. Pediatr Pulmonol 2013;48:419-42. [PubMed]
- Zhang XY, Simpson JL, Powell H, et al. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clin Exp Allergy 2014;44:1137-45. [PubMed]
- Katz LE, Gleich GJ, Hartley BF, et al. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc 2014;11:531-6. [PubMed]
- Spector SL, Tan RA. Is a single blood eosinophil count a reliable marker for “eosinophilic asthma?”. J Asthma 2012;49:807-10. [PubMed]
- Abd El-Motaleb GS, Abou Amer AA, Elawa GM, et al. Study of urinary leukotriene E4 levels in children with acute asthma. Int J Gen Med 2014;7:131-5. [PubMed]
- Rabinovitch N, Graber NJ, Chinchilli VM, et al. Urinary leukotriene E4/exhaled nitric oxide ratio and montelukast response in childhood asthma. J Allergy Clin Immunol 2010;126:545-51.e1-4.
- Parulekar AD, Atik MA, Hanania NA. Periostin, a novel biomarker of TH2-driven asthma. Curr Opin Pulm Med 2014;20:60-5. [PubMed]
- Wedes SH, Wu W, Comhair SA, et al. Urinary bromotyrosine measures asthma control and predicts asthma exacerbations in children. J Pediatr 2011;159:248-55. [PubMed]
- Ferrini ME, Simons BJ, Bassett DJ, et al. S-nitrosoglutathione reductase inhibition regulates allergen-induced lung inflammation and airway hyperreactivity. PloS One 2013;8:e70351. [PubMed]
- Basu S. Bioactive eicosanoids: role of prostaglandin F(2α) and F2-isoprostanes in inflammation and oxidative stress related pathology. Mol Cells 2010;30:383-91. [PubMed]
- Morris CR. Arginine and asthma. Nestle Nutr Inst Workshop Ser 2013;77:1-15. [PubMed]
- Sy HY, Ko FW, Chu HY, et al. Asthma and bronchodilator responsiveness are associated with polymorphic markers of ARG1, CRHR2 and chromosome 17q21. Pharmacogenet Genomics 2012;22:517-24. [PubMed]
- Smith AD, Cowan JO, Filsell S, et al. Diagnosing Asthma Comparisons between Exhaled Nitric Oxide Measurements and Conventional Tests. Am J Respir Crit Care Med 2004;169:473-8. [PubMed]
- Berkman N, Avital A, Breuer R, et al. Exhaled nitric oxide in the diagnosis of asthma: comparison with bronchial provocation tests. Thorax 2005;60:383-8. [PubMed]
- Matsunaga K, Hirano T, Akamatsu K, et al. Exhaled nitric oxide cutoff values for asthma diagnosis according to rhinitis and smoking status in Japanese subjects. Allergol Int 2011;60:331-7. [PubMed]
- Ren XB, Liu CT, Huang YF, et al. The diagnostic value of the fractional exhaled nitric oxide for asthma. Chinese Journal of Respiratory and Critical Care Medicine 2009;8:322-6.
- Sha L, Cao L, Ma Y, et al. Primary investigation of fractional concentration of exhaled nitric oxide in asthmatic children. Chinese Journal of Practical Pediatrics 2011;26:264-8.
- Rosi E, Ronchi MC, Grazzini M, et al. Sputum analysis, bronchial hyperresponsiveness, and airway function in asthma: results of a factor analysis. J Allergy Clin Immunol 1999;103:232-237. [PubMed]
- Dupont LJ, Demedts MG, Verleden GM. Prospective evaluation of the validity of exhaled nitric oxide for the diagnosis of asthma. Chest 2003;123:751-6. [PubMed]
- Schneider A, Tilemann L, Schermer T, et al. Diagnosing asthma in general practice with portable exhaled nitric oxide measurement--results of a prospective diagnostic study: FENO < or = 16 ppb better than FENO < or =12 ppb to rule out mild and moderate to severe asthma Respir Res 2009;10:15. [added]. [PubMed]
- Arora R, Thornblade CE, Dauby PA, et al. Exhaled nitric oxide levels in military recruits with new onset asthma. Allergy Asthma Proc 2006;27:493-8. [PubMed]
- Warke TJ, Fitch PS, Brown V, et al. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax 2002;57:383-7. [PubMed]
- Payne DN, Adcock IM, Wilson NM, et al. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med 2001;164:1376-81. [PubMed]
- Mattes J, Storm VGK, Reining U, et al. NO in exhaled air is correlated with markers of eosinophilic airway inflammation in corticosteroid-dependent childhood asthma. Eur Respir J 1999;13:1391-5. [PubMed]
- Jatakanon A, Lim S, Kharitonov SA, et al. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax 1998;53:91-5. [PubMed]
- Jones SL, Kittelson J, Cowan JO, et al. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med 2001;164:738-43. [PubMed]
- Berry MA, Shaw DE, Green RH, et al. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy 2005;35:1175-9. [PubMed]
- Li R, Zhao HJ, Cai SX, et al. Correlation between exhaled nitric oxide and lung function, ACQ7 scores in patients with bronchial asthma. Chinese Journal of Asthma 2012;6:151-5. (Electronic Version).
- Gong Y, Ye L, An X, et al. Roles of fractional exhaled nitric oxide (FeNO) in the management of bronchial asthma. Fudan University Journal of Medical Sciences 2013;40:349-53.
- Strunk RC, Szefler SJ, Phillips BR, et al. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol 2003;112:883-92. [PubMed]
- Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:231-7. [PubMed]
- Porsbjerg C, Lund TK, Pedersen L, et al. Inflammatory subtypes in asthma are related to airway hyperresponsiveness to mannitol and exhaled NO. J Asthma 2009;46:606-12. [PubMed]
- Westergaard CG, Munck C, Helby J, et al. Predictors of neutrophilic airway inflammation in young smokers with asthma. J Asthma 2014;51:341-7. [PubMed]
- Amelink M, de Groot JC, de Nijs SB, et al. Severe adult-onset asthma: a distinct phenotype. J Allergy Clin Immunol 2013;132:336-41. [PubMed]
- Silkoff PE, Lent AM, Busacker AA, et al. Exhaled nitric oxide identifies the persistent eosinophilic phenotype in severe refractory asthma. J Allergy Clin Immunol 2005;116:1249-55. [PubMed]
- Szefler SJ, Martin RJ. Lessons learned from variation in response to therapy in clinical trials. J Allergy Clin Immunol 2010;125:285-92, 293-4. [PubMed]
- Smith AD, Cowan JO, Brassett KP, et al. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med 2005;172:453-9. [PubMed]
- Szefler SJ, Martin RJ, King TS, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol 2002;109:410-8. [PubMed]
- Knuffman JE, Sorkness CA, Lemanske RJ, et al. Phenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthma. J Allergy Clin Immunol 2009;123:411-6. [PubMed]
- Anderson WJ, Short PM, Williamson PA, et al. Inhaled corticosteroid dose response using domiciliary exhaled nitric oxide in persistent asthma: the FENOtype trial. Chest 2012;142:1553-61. [PubMed]
- Nishio K, Odajima H, Motomura C, et al. Effect of inhaled steroid therapy on exhaled nitric oxide and bronchial responsiveness in children with asthma. J Asthma 2006;43:739-43. [PubMed]
- Jatakanon A, Lim S, Chung KF, et al. An inhaled steroid improves markers of airway inflammation in patients with mild asthma. Eur Respir J 1998;12:1084-8. [PubMed]
- Taylor DR. Advances in the clinical applications of exhaled nitric oxide measurements. J Breath Res 2012;6:047102. [PubMed]
- Cowan DC, Cowan JO, Palmay R, et al. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax 2010;65:384-90. [PubMed]
- Plaza V, Ramos-Barbon D, Munoz AM, et al. Exhaled nitric oxide fraction as an add-on to ACQ-7 for not well controlled asthma detection. PLoS One 2013;8:e77085. [PubMed]
- Yoon JY, Woo SI, Kim H, et al. Fractional exhaled nitric oxide and forced expiratory flow between 25% and 75% of vital capacity in children with controlled asthma. Korean J Pediatr 2012;55:330-6. [PubMed]
- Kharitonov SA, Donnelly LE, Montuschi P, et al. Dose-dependent onset and cessation of action of inhaled budesonide on exhaled nitric oxide and symptoms in mild asthma. Thorax 2002;57:889-96. [PubMed]
- Pérez-de-Llano LA, Carballada F, Castro Añón O, et al. Exhaled nitric oxide predicts control in patients with difficult-to-treat asthma. Eur Respir J 2010;35:1221-7. [PubMed]
- Beck-Ripp J, Griese M, Arenz S, et al. Changes of inhaled nitric oxide during steroid treatment of childhood asthma. Eur Respir J 2002;19:1015-9. [PubMed]
- Koster ES, Raaijmakers JA, Vijverberg SJ, et al. Inhaled corticosteroid adherence in paediatric patients: the PACMAN cohort study. Pharmacoepidemiol Drug Saf 2011;20:1064-72. [PubMed]
- McNicholl DM, Stevenson M, McGarvey LP, et al. The utility of fractional exhaled nitric oxide suppression in the identification of nonadherence in difficult asthma. Am J Respir Crit Care Med 2012;186:1102-8. [PubMed]
- Asthma Assembly of Chinese Thoracic Society. Guideline of asthma management (strategy of definition, diagnosis, treatment and management). Chinese Journal of Tuberculosis and Respiratory Diseases 2008;31:177-85.
- Soto-Ramos M, Castro-Rodríguez JA, Hinojos-Gallardo LC, et al. Fractional exhaled nitric oxide has a good correlation with asthma control and lung function in latino children with asthma. J Asthma 2013;50:590-4. [PubMed]
- Dweik RA, Sorkness RL, Wenzel S, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med 2010;181:1033-41. [PubMed]
- Michils A, Baldassarre S, Van Muylem A. Exhaled nitric oxide and asthma control:a longitudinal study in unselected patients. Eur Respir J 2008;31:539-46. [PubMed]
- Zeiger RS, Schatz M, Zhang F, et al. Elevated exhaled nitric oxide is a clinical indicator of future uncontrolled asthma in asthmatic patients on inhaled corticosteroids. J Allergy Clin Immunol 2011;128:412-4. [PubMed]
- Gelb AF, Flynn Taylor C, Shinar CM, et al. Role of spirometry and exhaled nitric oxide to predict exacerbations in treated asthmatics. Chest 2006;129:1492-9. [PubMed]
- Jayaram L, Pizzichini MM, Cook RJ, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J 2006;27:483-94. [PubMed]
- Smith AD, Cowan JO, Brassett KP, et al. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med 2005;352:2163-73. [PubMed]
- Pijnenburg MW, Bakker EM, Hop WC, et al. Titrating steroids on exhaled nitric oxide in children with asthma: a randomized controlled trial. Am J Respir Crit Care Med 2005;172:831-6. [PubMed]
- Donohue JF, Jain N. Exhaled nitric oxide to predict corticosteroid responsiveness and reduce asthma exacerbation rates. Respir Med 2013;107:943-52. [PubMed]
- Mahr TA, Malka J, Spahn JD. Inflammometry in pediatric asthma: a review of fractional exhaled nitric oxide in clinical practice. Allergy Asthma Proc 2013;34:210-9. [PubMed]
- Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet 2011;378:983-90. [PubMed]
- Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet 2008;372:1065-72. [PubMed]
- de Jongste JC, Carraro S, Hop WC, et al. Daily telemonitoring of exhaled nitric oxide and symptoms in the treatment of childhood asthma. Am J Respir Crit Care Med 2009;179:93-7. [PubMed]
- Gibson PG. Using fractional exhaled nitric oxide to guide asthma therapy: design and methodological issues for Asthma Treatment Algorithm studies. Clin Exp Allergy 2009;39:478-90. [PubMed]
- Pijnenburg MW, Hofhuis W, Hop WC, et al. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax 2005;60:215-8. [PubMed]
- Zacharasiewicz A, Wilson N, Lex C, et al. Clinical use of noninvasive measurements of airway inflammation in steroid reduction in children. Am J Respir Crit Care Med 2005;171:1077-82. [PubMed]