
Outcome of parapneumonic empyema managed surgically or by fibrinolysis: a multicenter study
Introduction
Parapneumonic empyema (PPE) is a common clinical condition that is associated with significant morbidity and mortality. It occurs in 20% to 60% of patients that develop pneumonia (1). Parapneumonic empyema include exudative American Thoracic Society (ATS-1), fibrinopurulent (ATS-2) and organized (ATS-3) stages (2). The current guidelines for the management of PPEs involve wide spectrum antibiotics and pleural drainage as a first step (2). Following pleural drainage, two situations exist: full lung re-expansion or persistence of loculations, which suggests the diagnosis of ATS-2/3 PPE (2). The management of ATS-2/3 PPE remains controversial. While some advocate early surgery to widely debride the pleural space and decorticate the lung, others believe surgery can be replaced by fibrinolysis (3-7). Arguments for early surgical management of PPE include a better pleural space control with a decreased risk of lung restriction on the long term as well as a lower likelihood of having to perform pleural decortication via open thoracotomy (4-8).
The concept of fibrinolysis is to inject intrapleural drugs to biologically break down loculations. Intrapleural administration of fibrinolytic agents, such as urokinase, streptokinase, tissue plasminogen activator (t-PA) and deoxyribonuclease (DNase) have been reported as superior to simple chest tube water flush to manage PPE (9). The MIST2 trial showed that t-PA/DNase combined therapy improved fluid drainage and reduced the frequency of surgical referral compared to drainage alone based on pleural chest X-ray opacity correction (7).
Five clinical studies with small sample sizes have compared fibrinolysis to video assisted thoracic surgery (VATS) decortication of PPE, four in pediatric and one in adult patient populations (10-14). There was no difference with these approaches regarding infection control and overall outcome. However, the length of hospital stays (LOS) seemed shorter after VATS than fibrinolysis although non-significant. Interestingly, none of those studies assessed the impact of therapy on the quality of pleural space control and the pulmonary restriction on the long term.
The aim of this study was to compare the outcome of a similar patient population judged operable with loculated PPE (ATS-2/3) managed over a 4-year period in two high volume thoracic surgery institutions, one with an early surgical approach and the other with a medical fibrinolysis approach. An emphasis on the pleural space control (Chest X-ray pleural opacity change before and after therapy) is performed. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-1083).
Methods
Study design
All patients managed for a PPE by the divisions of thoracic surgery of the Lausanne and Geneva University Hospitals between January 2014 and 2018 were reviewed. Informed consent was obtained for all patients of this study. The data was collected in a common database. The study was approved by the local ethical committee and was accepted by the state of Geneva and the state of Vaud (Project-ID 2020-00181). The Thoracic University Centers of Geneva and Lausanne are located 45 kilometers apart and have recently fused into one center: the Thoracic Surgery University Center of Western Switzerland. The management policies for PPE were different in each center while the study population can be considered similar given the geographical proximity, access to health care, infection microbiology and co-morbidity characteristics. The study was performed in accordance with the Declaration of Helsinki (as revised in 2013).
Patients
All patients included in this study were judged operable (Karnofsky performance status score of 60 to 80 with no major cardiovascular co-morbidities) and diagnosed with PPE based on Chest CT, inflammation parameters in the bloodwork, pleural fluid contents (LDH, pH and Glucose) and pleural fluid cultures. Patients with pleural effusions originating from neoplasia, following surgery or related to chronic infection (i.e., mycobacterium tuberculosis) were excluded from this study.
Management of PPE
All patients initially received empirical antibiotics that consisted in Ceftriaxone + Clarithromycin or Piperacilline/Tazobactam which was tailored to the risk of pseudomonas aeruginosa pathogen (i.e., chronic obstructive pulmonary disease patients). Pleural effusions were drained in all cases either by interventional radiologists or by thoracic surgeons using a 10F or superior size pigtail under ultrasounds (US) or computerized tomography scan (CT-scan) guidance. Sputum cultures were performed in addition to pleural fluid cultures and urines were screened for Legionella and Pneumococcus antigens. Antibiotics were adapted to the cultures when a clear germ was identified.
Treatment was initiated following drainage in case of persistent pleural loculations based on chest X-ray or CT-scan: two different management approaches were performed in each center.
At the University Hospital of Lausanne, patients underwent early surgical debridement and decortication of the pleural cavity. The surgical approach consisted in a 3-port anterior approach with patients placed on general anesthesia with a double lumen tube. A first step consisted in lung liberation from the chest wall, the mediastinum and the diaphragm followed by pleural decortication. For this particular step, a continuous positive airway pressure device was placed on the excluded lung with a pressure of 5 to 10 cm of water to facilitate lung decortication. The lung was cleared from all fibrous tissue, pus and septa, the fissures were open, the diaphragm was freed and the obliteration of the space by the decorticated lung was checked for all cases. We did not used epidural analgesia. The surgery was initiated as VATS for each case and converted to thoracotomy when necessary. Two large 28CH chest tubes were placed in the pleural cavity at the end of the procedure.
At the University Hospital of Geneva, intrapleural fibrinolysis was carried out through the chest tube/pigtail. Between January 2014 and November 2016, all patients were treated with intrapleural Urokinase at a dosage of 250,000 units in 30 mL of 0.9% NaCl. This treatment was performed twice a day for 5 days. From December 2016, the fibrinolysis drugs were switched to intrapleural t-PA/DNase (10 mg t-PA and 5 mg DNase in 30 mL of 0.9% NaCl) injected twice a day for 3 days. After injection of the fibrinolytics, the chest drainage procedure was identical for both groups: the chest tube was rinsed with 20 mL of 0.9% NaCl, closed for 3 hours and then opened in a water-seal system with a suction of −20 cmH2O. Follow-up by X-ray and fluid recovery was performed during the treatment period. In case of persistent pleural opacity on chest X-ray at day 7, a chest CT scan was performed and remaining pleural collections were drained by interventional radiology. If infection parameters persisted or important pleural loculations remained, surgical management of the PPE was initiated.
Endpoints
For each patient, we recorded the morbidity, mortality, PPE treatment failure and additional procedures required, germ identification and type, inflammatory syndrome, C-reactive protein level, CRP, and Leucocyte counts before treatment initiation (surgery or fibrinolysis) and at the end of the treatment (day of discharge, if before day 7, or day 7). Patient co-morbidities were also recorded. For each patient, we also assessed the percentage of lung area occupied by pleural opacity on chest X-ray before treatment initiation and at the end of the treatment (day of discharge, if before day 7 or day 7). All chest X-rays used for this part of the study were performed on patients either standing up or sitting in the upright position (no X-rays in bed with lying patients). For this, we choose an approach that was similar to the MIST2 trial (7). Two blinded medical doctors assessed the pleural opacity over time and the results were pooled (Figure 1). This allowed an objective quantification of pleural opacity change over time. In the surgical group, we deliberately chose to compare discharge X-ray and not immediate postoperative X-ray to avoid bias caused by important lung recruitment with positive airway pressure.
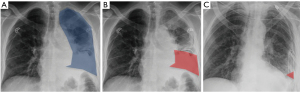
Chest tube duration and length of stay were assessed from the intervention (surgery or fibrinolysis) to the last chest tube removal (including pigtails or additional drains that were placed after surgery of fibrinolysis).
Chest tubes were removed in the surgical group when pleural fluid over 24 hours was inferior to 200 mL with no air flow. In the fibrinolytic group, similar criteria were applied but from the day following the termination of fibrinolysis.
We finally assessed the development of complications over the course of treatment. We defined hemothoraces as an excessive chest tube hematic output of more than 100 mL for two consecutive hours.
Statistical analysis
Continuous data are presented as mean [standard deviation (SD)] or median [interquartile range (IQR)] and categorical data as frequency with percentage. We compared patients’ characteristics and post-operative outcomes with unpaired Student’s t-tests or Mann-Whitney U tests for continuous variables. Student T test was applied with a bilateral hypothesis in a two-sided way. Categorical variables were tested by the χ2 test or Fisher’s exact test. Statistical analyses were performed using STATA 14 (StataCorp., TX, USA).
Results
We identified 66 patients that underwent surgical and 93 that underwent fibrinolytic management of their PPEs during the study period. The clinical and laboratory characteristics of both groups are reported in Table 1. While there was no difference in gender, side of affection, initial CRP or positive pleural fluid culture between both groups, patients from the surgical group were younger compared to fibrinolytic group (P=0.048), they had a higher day-0 leucocyte count (P=0.006). The co-morbidities were mild, similar between both groups and did not affect patient operability (Table 2). The pleural fluid characteristics are reported in Table 3. Pleural fluid showed pH<7.2 and/or positive culture and/or elevated levels of LDH or low glucose content in all patients, which was compatible with PPE. Surgery was performed by VATS in all cases within 2±2 days (0–6 days) following patient drainage while fibrinolysis was initiated at time of drainage. Surgery rate of conversion from VATS to open was of 15% (N=10). Interestingly, we observed no surgical conversion from VATS to thoracotomy during the last 2 years of the study period (from 2016 to 2018). We used a 3-port VATS anterior approach with a mean operative time of 122±55 minutes with a range of (27–255 minutes). The ATS classification could be accurately determined in the 66 patients of the surgical group with 42 patients (63.6%) that had ATS-2 and 24 patients (36.4%) that had ATS-3.
Table 1
| Variable | Surgery, N=66 | Fibrinolysis, N=93 | P value |
|---|---|---|---|
| Age (years), mean ± SD [range] | 56±16 [17, 84] | 62±17 [23, 94] | 0.048 |
| Gender (male, %) | 46 (69.7) | 64 (68.8) | 0.906 |
| Side (right, %) | 38 (57.6) | 56 (60.2) | 0.739 |
| Leukocytes day 0 (G/L), mean ± SD [range] | 18.2±8.6 [6, 48] | 14.9±6.4 [5, 45] | 0.006 |
| CRP day 0 (mg/L), mean ± SD [range] | 202.8±125.2 [18, 532] | 205.2±106.8 [16, 478] | 0.895 |
| Germ identification (%) | 28 (42.4) | 33 (35.5) | 0.375 |
CRP, C-reactive protein; SD, standard deviation.
Table 2
| Co-morbidity | Surgery (N=66)* | Fibrinolysis (N=67)* | P value |
|---|---|---|---|
| Systemic hypertension | 22 (33.3) | 29 (43.3) | 0.248 |
| Past history of ischemic cardiopathy | 11 (16.7) | 7 (10.5) | 0.295 |
| Past history of rhythmic cardiopathy | 8 (12.1) | 8 (12.0) | 0.916 |
| Chronic obstructive pulmonary bronchopathy | 8 (12.1) | 7 (10.5) | 0.705 |
| Diabetes mellitus | 6 (9.1) | 14 (20.9) | 0.057 |
| Nephropathy | 5 (7.6) | 7 (10.5) | 0.563 |
| Ethylism | 12 (18.2) | 8 (11.9) | 0.314 |
| Transient ischemic attack/stroke | 0 (0) | 4 (6.0) | 0.119 |
| Dementia | 0 (0) | 4 (6.0) | 0.119 |
| Oncological history | 10 (15.1) | 12 (17.9) | 0.669 |
*, co-morbidities are available in 133 of 159 patients.
Table 3
| Variable | Surgery | Fibrinolysis |
|---|---|---|
| pH, mean ± SD [range] | 7.0±0.4 [6.6–7.6] | 6.9±0.6 [6.1–7.4] |
| Glucose (mmol/L), mean ± SD [range] | 2.0±2.2 [0.1–6.6] | 2.3±2.4 [0.1–10.4] |
| Lactate deshydrogenase (LDH, /L), mean ± SD [range] | 2,269±2,339 [56–6,487] | 2,231±2,674 [42–13,206] |
| Germ identification | 42.4% | 35.5% |
| Enterobacteriaceae | 0 | 6% |
| Oro-dental | 12% | 4% |
| Streptococcus | 27% | 16% |
| Staphylococcus | 3% | 4% |
| Pseudomonas | 0 | 2% |
| Haemophilus | 0 | 2% |
Concerning the biological outcome, day 7 inflammatory parameters (Leucocyte count and CRP levels) were normalized and infection control was achieved in all patients from both groups (Figure 2). Chest X-ray pleural opacity percentage of the lung surface decreased significantly more with therapy in the surgical compared to the fibrinolytic group (−22%±18% vs. −16%±17% respectively, P=0.035, Table 4).
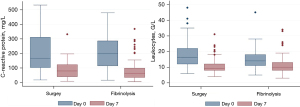
Table 4
| Variable | Surgery, N=66 | Fibrinolysis, N=93 | P value |
|---|---|---|---|
| Pleural effusion area changes at day7 (%), mean ± SD [range] | −22±18 [−80, 6] | −16±17 [−71, 12] | 0.035 |
| Additional drain (%) | 3 (4.6) | 20 (21.5) | 0.003 |
| Referral to surgery or redo (%) | 2 (3) | 12 (12.9) | 0.03 |
| Hemothorax (%) | 2 (3.1) | 0 (0) | 0.17 |
| Arrhythmia (%) | 4 (6.1) | 0 (0) | 0.027 |
| Drainage duration, median [IQR] | 3 [2–4] | 5 [4–7] | <0.0001 |
| Hospital length of stay, median [IQR] | 7 [5–10] | 11 [7–19] | <0.0001 |
IQR, interquartile range.
Persistent pleural collections were more often observed in the fibrinolytic group which required an additional drainage (4.6% vs. 21.5%, P=0.003 in the surgical and fibrinolytic group respectively, Table 4). Arrhythmia was, on the contrary, more frequent in the surgical group (6.1% vs. 0%, P=0.027 in the surgical and fibrinolytic group respectively). The latter were all treated pharmacologically (Clavien-Dindo Grade II complication). A highly hematic chest tube output occurred in 9 patients of the fibrinolytic group (9.6%). All patients with this complication had been treated by TPa-DNAse fibrinolysis and were successfully managed by treatment interruption and chest X-ray observation. No additional treatment was required for this complication (Clavien-Dindo Grade I). Referral to surgery was required in 12 patients of the fibrinolytic group (12.9%) and could be performed by VATS in all cases. Two patients (3%) of the surgical group required re-operation to manage a postoperative hemothorax (Clavien-Dindo Grade IIIB complication). Finally, 4 patients from the surgical group developed persistent air leak (more than 7 days) that were managed by Heimlich Valve placement and where chest tubes could all be removed by postoperative day 15 (Clavien-Dindo Grade I complication). There was no 30-day mortality and none of the patients required intensive care unit management during their entire hospital stay.
Chest tube drainage duration and length of hospital stay were shorter in the surgical group compared to the fibrinolytic group {3 [2–4] vs. 5 [4-7] days, P<0.0001 and 7 [5–10] vs. 11 [7–19] days, P<0.0001, respectively, Table 4}.
Discussion
Parapneumonic empyema is a frequent complication that requires appropriate management to control infection and pleural space. In the past, surgical therapy was more likely to be performed to avoid lung restriction (8). However, non-operative management approaches of PPE have improved over the past years with new molecules and protocols and interesting results (7-9).
In this retrospective study, we focused on the management of stage (ATS-2/3) PPEs diagnosed after initial drainage (persistent loculations) of operable patients (Karnofsky performance status of 60 to 80 and absence of major co-morbidities) managed either by surgery or fibrinolysis. The proximity of the two centers involved in this multicenter study and our patient characteristic analysis confirms that both patient populations were comparable. The infection was controlled in every case of both treatment groups (Leucocyte count and CRP). Pleural space was better controlled by surgery compared to fibrinolysis (pleural opacity change on chest X-ray). In the fibrinolytic group, patients required additional drains or a surgical procedure for definitive management in 21.5% and 12.5% of patients respectively. The median hospital stay and drainage duration were significantly shorter in the surgical compared to the fibrinolytic group. However, surgical therapy was associated with more complications (arrhythmias, hemothoraces and air leak). We observed a 15% conversion rate from VATS to thoracotomy which could illustrate our VATS learning curve as no conversions were recorded in the last two years of our study. Also, hospital stay was short (5 to 10 days) and little complications occurred.
We did find a significant difference in the mean age of patients between (6 years younger in the surgery group) between treatments. While patients were all judged operable, it is possible that more advanced ages in the fibrinolytic group may have affected decision to undergo surgery in case of partial lung re-expansion. However, this did not affect infection control outcome.
The role for VATS decortication in PPE has changed over the past decade. It was mostly used as a diagnosis procedure in between drainage and formal thoracotomy decortication. However, the improvement of minimal invasive surgery skills of surgeons over the past years have allowed most PPE decortications by VATS with less morbidity and mortality compared to standard thoracotomy. Five clinical trials compared the success rate (fluid drainage with no additional drain, mortality and infection control) of >ATS-2 stage empyema between fibrinolysis and VATS procedure (10-14). However, only one was conducted in adults patients with a low sample size (N=20) and they used Streptokinase (14). The authors found, as we did, that VATS was associated with a shorter hospital duration. Our study is in accordance with the literature. Despite a low level of evidence, a VATS approach is recommended in the first-line therapy in current surgical guidelines (2). However, a recent consensus proposed that intrapleural tPA and DNase can be used as either initial or subsequent therapy, depending on local expertise and the availability of minimally invasive surgical services (15). Pleural fibrinolysis has been widely studied: various molecules, approaches and vectors (high vs. low diameter tubes) have been reported (9). In the MIST2 trial, intrapleural tPAse/DNAse improved drainage of loculated pleural effusions, improved pleural space control on chest X-ray and decreased the hospital stay duration (7). Interestingly, high dose of intrapleural urokinase could be as efficient as tPA/DNAse treatment and could be associated with a lower rate of complications (16). In this study, we found that fibrinolysis with modern protocols (2–3 injections per day, tPase/DNAse molecules) was efficient in controlling PPE infection but was inferior in regards to length of stay, drainage duration and pleural space control. While the fibrinolysis treatment protocol changed over time, we did not observe an impact on the radiological and biological outcomes of these patients with one or the other approach.
With the current improvements of fibrinolytic approaches and the capacity of most surgeons to deal with advanced PPEs by VATS, the question of when to apply each therapeutic approach in PPE remains open. Some studies have shown that a 5-day trail of fibrinolysis did not impair the possibility to perform PPE decortication by VATS while others have suggested that surgical management delay increased the risk of thoracotomy (4,5). In our study, 12.5% of patients required surgery for definitive PPE management. Similarly to prior study, the delay for fibrinolysis did not impair VATS management of PPE in all patients (7). Many factors are taken into consideration for a change in PPE management strategy. Infection control, general state of the patient and X-ray change with treatment. Interestingly, although surgery could be applied in case of an insufficient effect of fibrinolysis, the pleural space was better controlled at the end of therapy in the surgical compared to the fibrinolytic group. This observation could be of importance when assessing the long term consequences of PPE on patient pulmonary function with potentially more pulmonary restriction in the patients with a poor pleural space correction. We had patient data up to 30 days after their discharge and did not observe recurrences, re-hospitalizations within this period. However, longer term assessments at 90 days and 1 year would be of interest regarding general activities, empyema recurrence and mortality. While this element is beyond the scope of this study, it would be an interesting parameter to assess in a clinical trial.
Two additional factors may have affected patient stay: the time between initial drainage and surgical management and the social, general state or rehabilitation requirement of patients. In the surgical hospital, access to the operation room for differed emergencies was possible within 24 to 48 hours. This may not be the case in other hospital structures. Regarding the delayed discharge in patients who have completed the treatment, there are more reasons such as the general state of the patient, social factors and rehabilitation requirement.
This multicentric study has some limitations. First, the study was multicentric and both protocols were not applied in both centers. Interestingly, the patient population was very similar including gender, side and C reactive protein baseline did not significantly differ between groups. Second, the fibrinolytic treatment changed during the study, but it was demonstrated that urokinase and t-PA/DNase therapy have the same efficacy, with similar clinical outcomes (16). Third, the criteria to convert in thoracotomy were not clearly protocoled, depending on the surgeon and changed during the study with the increase of the skills in VATS technique.
Conclusions
Surgical management of ATS-2/3 PPEs lead to shorter hospital, chest tube durations and a better pleural space control based on chest X-ray compared to fibrinolysis. There was no difference in infection control and mortality. Prospective randomized studies comparing both approaches are mandatory.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-1083
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-1083
Peer Review File: Available at https://dx.doi.org/10.21037/jtd-21-1083
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-1083). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was obtained for all patients of this study. The data was collected in a common database. The study was approved by the local ethical committee and was accepted by the state of Geneva and the state of Vaud (Project-ID 2020-00181). The study was performed in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis 2007;45:1480-6. [Crossref] [PubMed]
- Shen KR, Bribriesco A, Crabtree T, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 2017;153:e129-46. [Crossref] [PubMed]
- Aboudara M, Maldonado F. Update in the Management of Pleural Effusions. Med Clin North Am 2019;103:475-85. [Crossref] [PubMed]
- Lardinois D, Gock M, Pezzetta E, et al. Delayed referral and gram-negative organisms increase the conversion thoracotomy rate in patients undergoing video-assisted thoracoscopic surgery for empyema. Ann Thorac Surg 2005;79:1851-6. [Crossref] [PubMed]
- Bouros D, Antoniou KM, Chalkiadakis G, et al. The role of video-assisted thoracoscopic surgery in the treatment of parapneumonic empyema after the failure of fibrinolytics. Surg Endosc 2002;16:151-4. [Crossref] [PubMed]
- Luh SP, Chou MC, Wang LS, et al. Video-assisted thoracoscopic surgery in the treatment of complicated parapneumonic effusions or empyemas: outcome of 234 patients. Chest 2005;127:1427-32. [Crossref] [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Abraham SV, Chikkahonnaiah P. Change in Pulmonary Function Following Decortication for Chronic Pleural Empyema. Turk Thorac J 2020;21:27-31. [Crossref] [PubMed]
- Altmann ES, Crossingham I, Wilson S, et al. Intra-pleural fibrinolytic therapy versus placebo, or a different fibrinolytic agent, in the treatment of adult parapneumonic effusions and empyema. Cochrane Database Syst Rev 2019;2019:CD002312 [Crossref]
- Cobanoglu U, Sayir F, Bilici S, et al. Comparison of the methods of fibrinolysis by tube thoracostomy and thoracoscopic decortication in children with stage II and III empyema: a prospective randomized study. Pediatr Rep 2011;3:e29 [Crossref] [PubMed]
- Marhuenda C, Barceló C, Fuentes I, et al. Urokinase versus VATS for treatment of empyema: a randomized multicenter clinical trial. Pediatrics 2014;134:e1301-7. [Crossref] [PubMed]
- Sonnappa S, Cohen G, Owens CM, et al. Comparison of urokinase and video-assisted thoracoscopic surgery for treatment of childhood empyema. Am J Respir Crit Care Med 2006;174:221-7. [Crossref] [PubMed]
- St Peter SD, Tsao K, Spilde TL, et al. Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: a prospective, randomized trial. J Pediatr Surg 2009;44:106-11; discussion 111. [Crossref] [PubMed]
- Wait MA, Sharma S, Hohn J, et al. A randomized trial of empyema therapy. Chest 1997;111:1548-51. [Crossref] [PubMed]
- Chaddha U, Agrawal A, Feller-Kopman D, et al. Use of fibrinolytics and deoxyribonuclease in adult patients with pleural empyema: a consensus statement. Lancet Respir Med 2021;9:1050-64. [Crossref] [PubMed]
- Bédat B, Plojoux J, Noel J, et al. Comparison of intrapleural use of urokinase and tissue plasminogen activator/DNAse in pleural infection. ERJ Open Res 2019;5:00084-2019. [Crossref] [PubMed]


