
Minimally invasive surgery in the management of resectable thymoma: a retrospective analysis from the National Cancer Database
Introduction
Thymoma is the most common primary thymic tumor. It is a rare slow growing neoplasm that occurs in 0.13 per 100,000-person years with a 5-year overall survival rate of 95% (1). Tumors without evidence of invasion into mediastinal structures should be resected upfront in order to avoid local growth, regional spread, or degeneration to a more malignant form (2-4). Current surgical approaches include median sternotomy, clamshell, thoracoscopy, and robot-assisted. The latter methods (i.e., minimally-invasive thymectomy) have gained increasing popularity due to reports of shorter length of stay (LOS), decreased post-operative pain, and lower operative blood loss (5). However, few reports exist on the oncologic efficacy of thoracoscopic or robotic approaches in the treatment of thymomatous tumors (6). Using the National Cancer Database (NCDB) which harbors 75% of all cancer cases in the United States, we aim to elucidate whether minimally-invasive thymectomy for thymoma have similar short-term and long-term oncologic outcomes as the open approach. We present the following article in accordance with the STROBE reporting checklist (available at
Methods
Data source
The NCDB is a large hospital-based registry and contains de-identified clinical data from oncologic resections from community, comprehensive community, and academic facilities in the United States. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Following an exemption granted by the Indiana University Institutional Review Board (IRB00000220, study #17091923470), all patients that were 18 years or older with the diagnosis of thymoma between January 1, 2010 through December 31, 2015 were selected and retrospectively analyzed. Any case that underwent preoperative therapy such as chemotherapy or radiotherapy were excluded from analysis. Only invasive cases were included in the analysis. Patients were divided into three cohorts for comparison: open (median sternotomy, clamshell, or thoracotomy), thoracoscopic [video-assisted thoracoscopic surgery (VATS)], and robotic-assisted thoracoscopic surgery (RATS). Any RATS or VATS cases that were converted to open were analyzed with their initial surgical cohort. Demographics including age, gender, and Charlson-Deyo Comorbidity Index were collected. Regional location and type of institution where the surgery was performed (community, comprehensive, academic, integrated) were also queried along with socioeconomic status including insurance status, highest level of education, and annual household income. Histopathological results including tumor size, histologic grade (WHO grading system), and extent of invasion were analyzed. Primary outcomes were margin status after resection (R0 resection) and utilization of postoperative adjuvant radiation. Secondary outcomes included 30- and 90-day mortality, hospital LOS (days), 30-day readmission rate (percentage), and 5-year overall survival.
Statistical analysis
Demographic variables between the groups were reported using descriptive statistics. Distribution of each continuous covariates were analyzed using Shapiro-Francia W test for normality (7). Medians (inter-quartile range) were reported for non-normal distributed data. Bivariate comparisons between the cohorts were performed using Pearson’s Chi-square tests for categorical variables and Kruskal Wallis rank tests for nonnormally distributed continuous variables (8). For sparsely populated categorical variables in bivariate analysis, with expected cell count <5, Fisher’s exact tests were reported. Penalized maximum likelihood multivariable logistic regressions (Firth’s method) were used for sparsely distributed 30- and 90-day mortality variables and maximum likelihood multivariable logistic regression models were used for R0 resection, LOS, 30-day readmissions, 5-year overall survival, extent of invasion, and adjuvant radiation (9,10). Multivariable regression analyses were performed to control for all demographics, facility type, socioeconomic status, comorbidities, histopathologic staging, and treatment variables.
Survival analysis
Using time from diagnosis to the last contact and/or death, we performed Kaplan-Meier (KM) analysis to estimate the survival function for the cohorts. Difference in survival between the cohorts were examined using log-rank and Wilcoxon-Breslow tests for equality of survivor functions. A cox regression for survival analysis was also performed.
Results
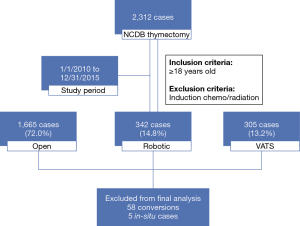
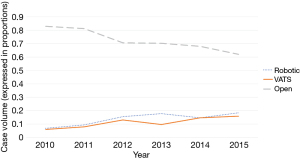
Between January 1, 2010 to December 31, 2015, 2,312 thymoma cases that underwent thymectomy as initial treatment were identified. In total, 1,665 cases (72.0%) were performed via open approach, 342 cases (14.8%) were robot-assisted, and 305 cases (13.2%) were performed using thoracoscopic methods (Figure 1). Seventeen RATS cases (5.0%) and 41 VATS cases (13.4%) were converted to open surgery. Between 2010 to 2015, RATS cases increased from 6.8% to 18.4% of total cases performed. VATS cases increased from 5.0% to 15.9%, while open cases decreased from 83% to 62.1% of total cases performed (Figure 2). For the simplicity of the analysis, 58 converted cases and five in-situ cases were dropped from the subsequent analysis.
Demographics
Among our three cohorts, the median age for open, RATS, and VATS were 61, 63, and 64 respectively (P<0.05). Caucasians were more commonly included in the study population than other races (P=0.017). There were no statistically significant differences in gender or Charlson-Deyo Comorbidity Index (Table 1). Comparisons of tumor size noted that patients in the RATS group had smaller thymomas when compared to VATS and sternotomy, respectively (45 versus 50 versus 61 mm, P<0.01) (Table 1). Socioeconomic factors were compared including high school education, median household income, and insurance status (Figure 3). The open group had a higher rate of uninsured patients and lower median household income when compared to RATS or VATS (Figure 2). The majority of RATS and VATS cases are being performed in academic centers in the Northeast and Southern region of the United States (P<0.0001). Open cases, on the other hand are equally distributed throughout all regions in the United States and the majority are done at comprehensive or academic institutions. Socioeconomic factors affected the type of operative approaches but did not have any meaningful impact on perioperative or oncology outcomes (Table 2).
Table 1
| Demographics | Open (N=1,661) | VATS (N=263) | Robotic (N=325) | P value |
|---|---|---|---|---|
| Age (years), median [range] | 61 [18–90] | 64 [20–88] | 63 [22–90] | 0.03** |
| Gender, n (%) | NS | |||
| Male | 815 (49.1) | 120 (45.6) | 151 (46.4) | |
| Female | 846 (50.9) | 143 (54.4) | 174 (53.5) | |
| Race, n (%) | 0.017 | |||
| Caucasian | 1,179 (71.0) | 207 (78.7) | 241 (74.2) | |
| African-American | 302 (18.2) | 40 (15.2) | 43 (13.2) | |
| Other | 167 (10.1) | 15 (5.7) | 37 (11.4) | |
| Charlson-Deyo Comorbidity Index, n (%) | NS | |||
| None | 1,198 (72.1) | 199 (75.7) | 225 (69.2) | |
| 1 | 366 (22.0) | 49 (18.6) | 84 (25.8) | |
| ≥2 | 97 (5.8) | 15 (5.7) | 16 (4.9) | |
| Tumor size (mm), median [range] | 61 [1–995] | 50 [5–990] | 45 [1–994] | <0.001** |
| WHO histologic grading, n (%) | 0.026 | |||
| Not otherwise specified (NOS) | 273 (16.4) | 36 (13.7) | 52 (16.0) | |
| A | 162 (9.8) | 25 (9.5) | 41 (12.6) | |
| AB | 328 (19.7) | 75 (28.5) | 74 (22.8) | |
| B1 | 191 (11.5) | 29 (11.0) | 41 (12.6) | |
| B2 | 263 (15.8) | 42 (16.0) | 58 (17.8) | |
| B3 | 229 (13.8) | 29 (11.0) | 27 (8.3) | |
| C | 215 (12.9) | 27 (10.3) | 32 (9.8) | |
| Invasion, n (%) | <0.001 | |||
| Localized | 802 (48.3) | 160 (60.8) | 196 (60.3) | |
| Capsular invasion | 336 (20.2) | 61 (23.2) | 79 (24.3) | |
| Mediastinal invasion | 496 (29.9) | 35 (13.3) | 46 (14.2) | |
| Radiation, n (%) | 0.002 | |||
| None | 1,005 (60.5) | 187 (71.1) | 212 (65.2) | |
| Adjuvant | 656 (39.5) | 76 (28.9) | 113 (34.8) | |
| Chemotherapy, n (%) | <0.0001 | |||
| None | 1,490 (89.7) | 242 (92.0) | 315 (96.9) | |
| Adjuvant | 171 (10.3) | 21 (8.0) | 10 (3.1) | |
| Conversion rate, n (%) | – | 41 (13.4) | 17 (5.0) | – |
**, Kruskal-Wallis test. WHO, World Health Organization; NS, not significant (P>0.05); VATS, video-assisted thoracoscopic surgery.
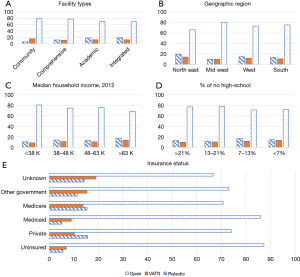
Table 2
| Variable | 30-day mortality* | 90-day mortality* | R0 resection** | 5-year mortality** | Adjuvant radiation** |
|---|---|---|---|---|---|
| Age | 0.95 | 0.97 | 1.01 | 1.04a | 0.98a |
| Female | 1.39 | 0.41 | 1.05 | 0.8 | 0.95 |
| Race—African American | 1.49 | 1.17 | 0.01 | 1.12 | 0.95 |
| Charlson-Deyo score | |||||
| 1 | 1.45 | 1.95 | 1.33 | 1.96a | 1.00 |
| ≥2 | 5.70a | 10.63a | 0.92 | 2.68a | 1.46 |
| Invasion/extension | |||||
| Capsular invasion | 1.25 | 1.42 | 1.72a | 1.51 | 2.60a |
| Mediastinal invasion | 2.00 | 2.07 | 3.30a | 2.61a | 2.67a |
| Histologic grade | |||||
| A | 2.08 | 3.79 | 0.53a | 1.35 | 0.63 |
| AB | 1.70 | 1.48 | 0.53a | 1.37 | 0.97 |
| B1 | 0.13 | 0.01 | 0.94 | 1.16 | 0.76 |
| B2 | 2.09 | 3.76 | 0.86 | 1.21 | 1.20 |
| B3 | 2.63 | 7.51a | 1.52 | 1.29 | 2.05a |
| C | 2.34 | 6.59 | 1.08 | 2.90a | 2.43a |
| Tumor size (9 cm+) | NS | NS | NS | 2.34a | NS |
| Operative approach | |||||
| VATS | 1.09 | 1.57 | 1.82a | 1.15 | 0.78 |
| Robotic | 0.26 | 0.37 | 0.89 | 1.81 | 0.98 |
| Adjuvant chemotherapy | – | – | 2.66a | 1.99a | – |
| Adjuvant radiation | – | 0.05a | 2.56a | 0.52a | – |
| Facility | |||||
| Comprehensive | 0.38 | 1.71 | 1.68 | 0.99 | 1.37 |
| Academic | 0.30 | 1.00 | 1.08 | 0.96 | 1.39 |
| Integrate | 0.09 | 0.24 | 1.52 | 0.96 | 1.79 |
| Region | |||||
| Midwest | 8.32 | 3.19 | 0.91 | 0.86 | 1.02 |
| West | 3.13 | 0.74 | 0.90 | 1.19 | 0.95 |
| South | 6.37 | 3.96 | 1.09 | 1.40 | 1.04 |
| Median income | |||||
| 38–48 K | 1.53 | 0.54 | 0.96 | 0.86 | 1.05 |
| 48–63 K | 3.14 | 1.26 | 1.06 | 1.00 | 0.90 |
| ≥63 K | 1.74 | 1.29 | 1.18 | 0.83 | 0.89 |
| Insurance status | |||||
| Private/managed care | 0.65 | 0.70 | 1.19 | 0.80 | 1.04 |
| Government/Medicare/aid | 1.95 | 3.66 | 1.08 | 1.48 | 1.02 |
| No high school education | NS | NS | NS | NS | NS |
a, statistically significant; *, firth-logistic regression; **, maximum likelihood logistic regression. NS, not significant (P>0.05); VATS, video-assisted thoracoscopic surgery.
Perioperative outcomes
Bivariate analyses noted no significant difference in 30- or 90-day mortality between all three cohorts. Open patients had a longer LOS (median 4 days) compared to RATS (2 days) and VATS (3 days) patients (P<0.01). There were no significant differences in LOS between the RATS and VATS group but there is a significant difference when comparing the LOS of the minimally-invasive to the open group (P<0.001). Ninety patients (5.4%) were readmitted within 30 days from the open group versus 11 patients (3.4%) from the RATS group and 11 patients (4.2%) from the VATS group (P>0.05) (Table 3).
Table 3
| Outcome measured | Open (N=1,661) | VATS (N=263) | Robotic (N=325) | P value |
|---|---|---|---|---|
| 30-day mortality, n (%) | 11 (0.7) | 3 (1.1) | 2 (0.6) | NS* |
| 90-day mortality, n (%) | 26 (1.6) | 3 (1.1) | 3 (0.9) | NS* |
| Length of stay, median | 4 | 3 | 2 | <0.001** |
| 30-day readmission, n (%) | 90 (5.4) | 11(4.2) | 11 (3.4) | NS |
| Mediastinal invasion, n (%) | 496 (29.9) | 35 (13.3) | 46 (14.2) | <0.001 |
| R0 resection, n (%) | 1,075 (64.7) | 166 (63.1) | 232 (71.4) | 0.032 |
*, Fisher’s exact test; **, Kruskal-Wallis test. NS, not significant (P>0.05); VATS, video-assisted thoracoscopic surgery.
Oncologic outcomes
Within the cohorts, the RATS group had a higher type A tumor than the VATS or open group (P<0.05). The VATS group had a lower proportion of tumors that are classified as not otherwise specified (NOS) than the other two groups (P<0.05) (Table 1). Robot-assisted thymectomy cohorts had a higher rate of complete R0 resection (negative margins) than the VATS and open group (71.4% versus 63.1% versus 64.7%, P=0.032). Similarly, the rate of mediastinal invasion was lower in RATS group (14.2%) as compared to open group (29.9%, P<0.001)) but not the VATS group (13.3%) (P>0.05) (Table 3). Conversely, more open patients underwent adjuvant radiation (39.5%) than RATS (34.8%) and VATS (28.9%) (Table 1). Multivariable regression analyses were performed to identify predictors of complete resection and adjuvant radiation. The presence of capsular [adjusted odds ratio (AOR) 1.72, 95% CI: 1.25–2.35] and mediastinal invasion (AOR 3.30, 95% CI: 2.42–4.5) was associated with incomplete resection. The presence of positive margins (AOR 2.63, 95% CI: 1.98–3.50) and mediastinal invasion (AOR 2.67, 95% CI: 2.04–3.51) were independent predictors for the use of adjuvant radiation. Different surgical approaches including open, RATS, and VATS were not independent predictors of R0 resection or the need for adjuvant radiotherapy (Table 2).
Survival
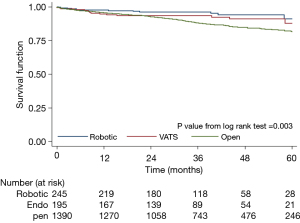
KM survival analyses were performed for all three cohorts. There were no differences in 5-year survival between the RATS and VATS group (P>0.05). Initially analysis noted a statistically significant difference between the RATS versus the open group (P<0.003) (Figure 4). However, the adjusted multivariate model in Cox regression analysis negated any significant differences when it comes to 5-year overall survival in all three groups (Table 2).
Discussion
Advances in minimally-invasive surgical techniques are constantly under scrutiny in terms of both safety and efficacy. In cases where few randomized controlled trials are available, analyses of large databases can provide information to guide current practices. Surgical resection of the thymus using thoracoscopic or robot-assisted methods have been shown to be safe with improved outcomes relating to LOS and pain (11-13). Minimally-invasive thymectomy also achieved similar perioperative outcomes such as surgical site infections and 30- and 90-day mortality (14-26). Data from these studies are heterogenous at best in that it included resections of benign or involuted thymus for symptomatic control of Myasthenia Gravis (14-28). However, few reports exist to determine whether minimally-invasive techniques have similar efficacy as sternotomy when looking at oncologic determinants such as operative margins (5,6,28,29). To this end, the NCDB provides a robust patient population for comparison. Our results suggest that both robot-assisted and thoracoscopic thymectomy for thymomatous tumors can be performed with similar perioperative and oncologic outcomes as traditional sternotomy.
For thymoma, open surgery, typically via midline sternotomy, has been the approach of choice especially for larger tumors or in cases of suspected mediastinal structure invasion. Based on our bivariate analyses, this holds true as RATS and VATS have smaller tumors and lower rate of extracapsular invasion on final pathology. This is likely responsible for the higher rate of R0 resection and lower rate of adjuvant radiation in the RATS and VATS group. However, our multivariable analyses noted that tumor size and robotic surgical approaches do not have a statistically significant association with negative margins. It is noteworthy to mention that the VATS approach is associated with increased odds ratio of a positive margin (OR 1.8, 95% CI: 1.15–2.88). However, it is unable to determine from this data if these are also the tumors that invade mediastinal structures noted at the time of the operation rather than known preoperatively. The one structure worthy of mentioning is the phrenic nerve as preoperative imaging are often times inadequate in assessing involvement. The tumors that involve such nerves may be small in size but the location may make it not amendable to achieve an R0 resection (2). As such, the intraoperative decision to leave the phrenic nerves intact may result in a higher rate of conversion or R1 resection. Regardless, the slight increase in OR for VATS approach does not affect 5-year overall survival. Overall, the data suggests that RATS and open approaches have an equal likelihood of achieving R0 resection despite the size of the tumor. The only factor that affects margin status is the presence of extracapsular extension with mediastinal invasion which likely prohibits complete dissection to preserve important mediastinal structures.
We decided to use adjuvant radiation as an additional marker for incomplete resection (30-33). In many cases of thymomas, wide margins cannot be obtained due to the presence of multiple vital structures within the mediastinum. It is not infrequent for the pathology report to mention margins that are negative but have tumor within 1 mm from the resection line. In some cases with minimally-invasive resections, the margins may be difficult to examine as the specimen may be compressed during extraction from the port site. For these reasons, adjuvant radiation may be utilized even with R0 resection in order to prevent local recurrences (30,32). In our analyses, more patients in the open group (39.4%) underwent adjuvant radiation than the RATS (34.2%) or VATS (30.8%) group, possibly due to higher likelihood of mediastinal invasion and a higher rate of positive margins. However, in multivariate analyses, surgical approaches are not associated higher rate of adjuvant radiation. This suggests that RATS and VATS can achieve similar oncologic results. In fact, positive margins and extracapsular extension were the only significant predictors of any adjuvant radiotherapy.
The analysis yielded several unexpected findings including the higher rate of conversion from VATS compared to RATS. The difference is thought to be due to the one-sided approach and the limited ability for the thoracoscopic camera to visualize the contralateral phrenic nerve (25). The robotic technology allows for carbon dioxide insufflation and rotational manipulation of the instrument arms which may lead to overall improvement in exposure and visualization. However, since the NCDB database does not provide any institution-, case-, or surgeon-specific data, it is difficult to prove that the learning curve or technological constraint is responsible for this difference. Secondly, evaluation of the impact of socioeconomic factors notes that more than 80% of uninsured and low-income patients underwent open thymectomy. Meanwhile, only 1% of patients in the RATS group are uninsured (versus 4% in the open group, P<0.05) and 11% have low-income status (versus 17% in the open group, P<0.05). Although Marulli et al. reported a potential decrease in costs associated with RATS due to shorter length of hospitalizations (17), the results from this socioeconomic analysis may highlight the perceived financial constraints in implementing a new treatment modality to patients with limited access to care.
Study limitations include data errors that are inherent to national databases. The retrospective nature of the study also subjects the data to recall, missing data, and selection bias. The rate of data missingness ranged from 0 to 7.8%. We assumed the data was completely at random and casewise deletion was therefore used. The NCDB does not differentiate a sternotomy from other open approaches (such as clamshell or open thoracotomy) thus the outcome data for the open group maybe be subjected to heterogeneity. Other limitations include the inability to determine recurrence-free survival data. In slow-growing indolent tumors such as thymoma where the 5-year overall survival rate approaches 95%, disease recurrence is a better variable to assess oncologic efficacy. Alternatively, we used margin status and adjuvant radiation as surrogate prognostic markers to determine oncologic effectiveness as previously described by Rea et al. In addition, we did not perform propensity-matched analyses as this would eliminate more than 60% of cases and drastically reduce the sample size, hence introducing type B errors. Lastly, our analyses did not consider if surgical volume plays a role in the outcome. Our bivariate analysis suggested that academic centers did perform more robotic resections. However, the effect of the type of institution was negated on multivariate analysis.
Conclusions
Robotic and thoracoscopic thymectomy have been suggested to have improved short-term outcomes as compared to gold-standard sternotomy. Our study of large national database suggests that the benefits extend beyond shorter LOS and decreased post-operative pain. RATS and VATS thymectomies have similar rate of complete resection and need for adjuvant radiation.
Acknowledgments
The abstract has been presented at the American College of Surgeons Clinical Congress 2019. (San Francisco, CA; October 28, 2019).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-20-2660
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-20-2660). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). An exemption was granted by the Indiana University Institutional Review Board (IRB00000220, study #17091923470).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260-5. [Crossref] [PubMed]
- Falkson CB, Bezjak A, Darling G, et al. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol 2009;4:911-9. [Crossref] [PubMed]
- Tomaszek S, Wigle DA, Keshavjee S, et al. Thymomas: review of current clinical practice. Ann Thorac Surg 2009;87:1973-80. [Crossref] [PubMed]
- Tseng YC, Hsieh CC, Huang HY, et al. Is thymectomy necessary in nonmyasthenic patients with early thymoma? J Thorac Oncol 2013;8:952-8. [Crossref] [PubMed]
- Kaufman AJ, Flores RM. Minimally invasive thymectomy for thymoma: does surgical approach matter or is it a question of stage? J Thorac Dis 2016;8:E1711-4. [Crossref] [PubMed]
- Burt BM, Yao X, Shrager J, et al. Determinants of Complete Resection of Thymoma by Minimally Invasive and Open Thymectomy: Analysis of an International Registry. J Thorac Oncol 2017;12:129-36. [Crossref] [PubMed]
- Royston P. Estimating departure from normality. Stat Med 1991;10:1283-93. [Crossref] [PubMed]
- Nahm FS. Nonparametric statistical tests for the continuous data: the basic concept and the practical use. Korean J Anesthesiol 2016;69:8-14. [Crossref] [PubMed]
- Coveney J. FIRTHLOGIT: Stata Module to Calculate Bias Reduction in Logistic Regression. 2008. [Last accessed on 2015 Jan 3]. Available online: http://econpapers.repec.org/software/bocbocode/s456948.htm
- Wang X. Firth logistic regression for rare variant association tests. Front Genet 2014;5:187. [Crossref] [PubMed]
- Friedant AJ, Handorf EA, Su S, et al. Minimally Invasive versus Open Thymectomy for Thymic Malignancies: Systematic Review and Meta-Analysis. J Thorac Oncol 2016;11:30-8. [Crossref] [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [Crossref] [PubMed]
- Curcio C, Scaramuzzi R, Amore D. Robotic-assisted thoracoscopic surgery thymectomy. J Vis Surg 2017;3:162. [Crossref] [PubMed]
- Hess NR, Sarkaria IS, Pennathur A, et al. Minimally invasive versus open thymectomy: a systematic review of surgical techniques, patient demographics, and perioperative outcomes. Ann Cardiothorac Surg 2016;5:1-9. [PubMed]
- Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81; discussion 981-2. [Crossref] [PubMed]
- Kneuertz PJ, Kamel MK, Stiles BM, et al. Robotic Thymectomy Is Feasible for Large Thymomas: A Propensity-Matched Comparison. Ann Thorac Surg 2017;104:1673-8. [Crossref] [PubMed]
- Marulli G, Comacchio GM, Schiavon M, et al. Comparing robotic and trans-sternal thymectomy for early-stage thymoma: a propensity score-matching study. Eur J Cardiothorac Surg 2018;54:579-84. [Crossref] [PubMed]
- Huang P, Ye B, Yang Y, et al. Experience with the "da Vinci" robotic system for early-stage thymomas: Report of 23 cases. Thorac Cancer 2014;5:325-9. [Crossref] [PubMed]
- Melfi F, Fanucchi O, Davini F, et al. Ten-year experience of mediastinal robotic surgery in a single referral centre. Eur J Cardiothorac Surg 2012;41:847-51. [Crossref] [PubMed]
- Mussi A, Fanucchi O, Davini F, et al. Robotic extended thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2012;41:e43-6; discussion e47. [Crossref] [PubMed]
- Rowse PG, Roden AC, Corl FM, et al. Minimally invasive thymectomy: the Mayo Clinic experience. Ann Cardiothorac Surg 2015;4:519-26. [PubMed]
- Rückert JC, Walter M, Müller JM. Pulmonary function after thoracoscopic thymectomy versus median sternotomy for myasthenia gravis. Ann Thorac Surg 2000;70:1656-61. [Crossref] [PubMed]
- Wang GW, Tao T, Li CK, et al. Comparison between thoracoscopic and open approaches in thymoma resection. J Thorac Dis 2019;11:4159-68. [Crossref] [PubMed]
- Youssef SJ, Louie BE, Farivar AS, et al. Comparison of open and minimally invasive thymectomies at a single institution. Am J Surg 2010;199:589-93. [Crossref] [PubMed]
- Zhang X, Gu Z, Fang W, et al. Minimally invasive surgery in thymic malignances: the new standard of care. J Thorac Dis 2018;10:S1666-70. [Crossref] [PubMed]
- Youssef SJ, Louie BE, Farivar AS, et al. Comparison of open and minimally invasive thymectomies at a single institution. Am J Surg 2010;199:589-93. [Crossref] [PubMed]
- Bachmann K, Burkhardt D, Schreiter I, et al. Long-term outcome and quality of life after open and thoracoscopic thymectomy for myasthenia gravis: analysis of 131 patients. Surg Endosc 2008;22:2470-7. [Crossref] [PubMed]
- Di Crescenzo VG, Napolitano F, Panico C, et al. Surgical approach in thymectomy: Our experience and review of the literature. Int J Surg Case Rep 2017;39:19-24. [Crossref] [PubMed]
- Odaka M, Tsukamoto Y, Shibasaki T, et al. Surgical and oncological outcomes of thoracoscopic thymectomy for thymoma. J Vis Surg 2017;3:54. [Crossref] [PubMed]
- Kim S, Bull DA, Hsu CH, et al. The Role of Adjuvant Therapy in Advanced Thymic Carcinoma: A National Cancer Database Analysis. Ann Thorac Surg 2020;109:1095-103. [Crossref] [PubMed]
- Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer 2002;94:624-32. [Crossref] [PubMed]
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84. [Crossref] [PubMed]
- Weis CA, Yao X, Deng Y, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol 2015;10:367-72. [Crossref] [PubMed]


