Histopathological findings in a critically ill patient with avian influenza A (H7N9)
Introduction
Many reports of fatal human infections with avian influenza A (H7N9) virus have emerged. However, the data regarding the pulmonary histopathology of human H7N9 disease are relatively rare: only a few autopsy cases have been reported (1-4) and pulmonary tissue samples from a living patient have yet to be assessed. We herein describe a patient with a severe and ultimately fatal case of avian influenza A (H7N9). While the patient was alive, pathological specimens from lung tissue were obtained by video-assisted thoracic surgery (VATS), and a limited autopsy of lung tissue was also performed by transbronchial lung biopsy (TBLB) after the patient’s death. All the histopathological results were analyzed.
Case presentation
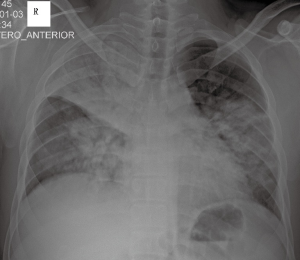
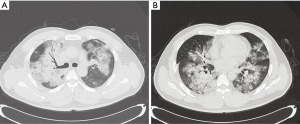
On 3 January 2015, a 28-year-old man was admitted to our respiratory intensive care unit (RICU) complaining of dyspnoea, fever, cough and phlegm with blood for 3 days. His past medical history was unremarkable. The patient’s travel history revealed that the patient had walked past some poultry stalls a few days prior to his symptom onset, but he did not have any direct contact with the poultry. The laboratory results showed increased hepatic- and cardiac-associated enzymes (alanine aminotransferase, aspartate amino transferase, gamma-glutamyl transpeptidase, creatine kinase, lactic dehydrogenase and alpha-hydroxybutyrate dehydrogenase). Leucopenia, lymphopenia and thrombocytopenia were also noted upon admission. The initial nasopharyngeal aspirate obtained on admission (hospital day 1) was positive for influenza A (H7N9) virus by reverse transcription PCR (RT-PCR), which was performed as a qualitative assay. A chest X-ray revealed multiple infiltrates in both lungs (Figure 1). A computed tomography scan confirmed these findings and showed diffuse ground-glass opacities and consolidation throughout the lungs (Figure 2A,B). Hypoxic respiratory failure and aggravated tachypnoea led to endotracheal intubation and mechanical ventilation on the first day of hospitalization. Even though the patient was treated with oseltamivir (150 mg twice daily), his condition continued to deteriorate. Extracorporeal membrane oxygenation (ECMO) was required on hospital day 5 (the 8th day after symptom onset). Bronchoalveolar lavage specimens suggested possible co-infection with three potential pathogens (Chryseobacterium indologenes, Candida albicans and Acinetobacter baumannii) and empiric antimicrobial therapy was administered. Due to the emergence of a right pneumothorax on hospital day 10 and a left pneumothorax on hospital day 15, chest tube drainage was successively performed into the cavum pleurae bilaterally; however, the right pneumothorax had a persistent air leak. Transbronchoscopic balloon detection and selective bronchial occlusion of the right intractable pneumothorax were performed on hospital days 16 and 17, respectively; however, the patient still failed to improve. Then, a resection of pulmonary bullae in the right middle lobe was performed by VATS on hospital day 20 (the 23rd day after symptom onset) and the air leakage ceased. The bronchoalveolar lavage fluid (BALF) specimen remained positive for influenza A (H7N9) virus by RT-PCR on hospital day 19 (the 22nd day after symptom onset); however, the specimen was negative for influenza A (N9) virus on hospital day 22 (the 25th day after symptom onset), although the specimen was still positive for influenza A (H7) virus at that time. The BALF specimens were tested again on hospital days 25 and 26 (the 28th and 29th days after symptom onset, respectively), and the results were negative for influenza A (H7N9) virus by RT-PCR. During the late phase of the clinical course, laboratory examinations revealed normal serum procalcitonin levels and white blood cell counts. Concurrently, repeat chest X-rays revealed that the initial large infiltrates and consolidation shadows had clearly resolved radiographically in both lungs. However, the patient’s clinical status continuously deteriorated with a progressive decline of lung compliance and refractory hypoxemia, which was considered to be due to progressive and rapid lung fibrosis. Finally, he developed multi-organ failure complicated by refractory hypoxemia and died on hospital day 36 (the 39th day after symptom onset), despite corticosteroid treatment. Due to religious reasons, the patient’s relatives only consented to a limited post-mortem biopsy and small samples of right lower lung tissue were obtained by TBLB after the patient’s death. The final histological findings showed diffuse severe pulmonary fibrosis, which was compatible with the clinical manifestations.
Histological findings
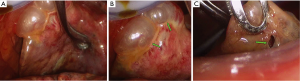
The findings of the right lung under VATS on the 23rd day after symptom onset were as follows: the external appearance of the right lung appeared contracted and the lung presented with diffuse alveolar consolidation (Figure 3A), particularly in the lower lobe; moreover, multiple subpleural pulmonary bullae were present in the upper lobe along with two apparently giant pulmonary bullae (Figure 3B, green arrows) in the middle lobe, one of which exhibited a crevasse (Figure 3C, green arrow).
The gross examination of specimens resected from the right middle lobe revealed the formation of multiple subpleural pulmonary bullae. The cut surface of the lung tissue appeared honeycombed and consolidated.
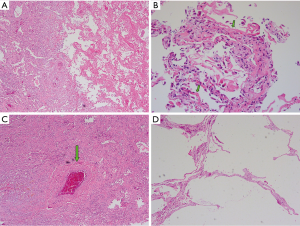
The pathohistological changes from the resected right middle lobe revealed diffuse alveolar damage and the remarkable findings were as follows: the lung tissue lesions appeared to be regionally distributed (Figure 4A) and included widened alveolar septae, inflammatory cell infiltration, intra-alveolar haemorrhage, diffuse interstitial pulmonary fibrosis and massive fibrinoid necrosis. Pulmonary oedema, hyperplasia of type II pneumocytes and hyaline membrane formation (Figure 4B, green arrows) were observed. Interstitial vascular proliferation, anapetia, hyperaemia and thrombus formation within the vascular lumen (Figure 4C, green arrow) were documented. Emphysema and pulmonary bulla formation (Figure 4D) could also be observed. Viral inclusions were not observed in the pathological specimens.
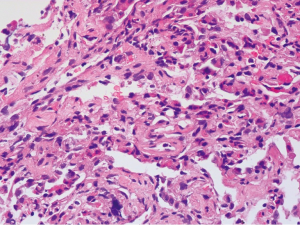
The patient died on the 39th day after symptom onset and a limited post-mortem biopsy of lung tissue was performed with small samples of right lower lung obtained by TBLB. The main pathohistological findings were as follows: hyperplasia and shedding of type II pneumocytes, severe pulmonary fibrosis, numerous lymphocytic infiltrates and only few neutrophilic infiltrates (Figure 5). Bronchial mucosal oedema and epithelial basement membrane thickening were documented. Hyaline thrombi within the vascular lumina were observed. Only a small amount of residual intra-alveolar hyaline membranes and intra-alveolar fibrous exudates were observed. Lung abscesses and viral inclusions were not observed.
Discussion
As recognized, illness resulting from severe infection with avian influenza A (H7N9) may be progressively aggravated within a brief time period after symptom onset. Patients with severe infections are prone to developing a variety of complications with a high mortality rate. In our patient, because of a persistent right pneumothorax air leak, a resection of the pulmonary bullae in the right middle lobe was performed by VATS based on the ECMO supportive technique on the 23rd day after symptom onset, after which the air leakage ceased. Diffuse alveolar consolidation and multiple pulmonary bullae were observed during the surgery and the resected lung specimens had a consolidated, honeycombed appearance. The major histopathological findings of the resected lung tissue were characterized by diffuse alveolar damage, intra-alveolar haemorrhage, hyaline membranes, pneumocyte hyperplasia and interstitial fibrosis, which are generally consistent with previous reports of influenza A (H7N9) (1-4). Additionally, these characteristics were similar to the results found in patients with influenza A (H1N1) (5) and avian influenza (H5N1) (6,7). Due to the pneumocyte hyperplasia and fibroproliferative changes in addition to the diffuse alveolar damage, the histopathological findings of the patient were compatible with the fibroproliferative phase. Additionally, when the patient died on the 39th day after symptom onset, a limited post-mortem biopsy of right lower lung tissue was performed by TBLB, revealing the histological changes of severe pulmonary fibrosis and clearly diminished acute exudative features. Notably, the lengthiest period from symptom onset to lung biopsy reported in patients with influenza A (H7N9) was 27 days (4); however, in our case the time of autopsy was 39 days after symptom onset, which was much longer than previously reported. The main histopathological features were those of pulmonary fibrosis; additionally, thrombus was observed in this patient. The thrombus was considered to be due to the severe infection, but also might have been caused by the ECMO therapy (2). Further studies are required to clarify the causes of thrombus formation in patients with influenza A (H7N9) virus infection.
Due to the laboratory limitations of our hospital, immunohistochemistry for influenza A viral antigen in the lung tissue specimens could not be performed. However, a previous report has shown that immunostaining for influenza A virus nucleoprotein (NP) expression was positive in the alveolar epithelium when the sputum sample was positive for influenza A (H7N9) virus by RT-PCR (1). Simultaneous with the nasopharyngeal aspirate and blood positivity for influenza A (H7N9) virus by RT-PCR, the pulmonary alveolar epithelial cells were also positive for influenza NP by immunohistochemistry (2). In fact, the literature demonstrates that the RT-PCR positive results for influenza A (H7N9) viral RNA in respiratory secretions should correlate well with the positive expression of influenza A viral antigen in the lung tissue of patients with viral pneumonia.
Notably in the present case, the BALF specimens were continuously positive for influenza A (H7N9) virus for more than three weeks after symptom onset, despite oseltamivir treatment. Due to the laboratory limitations, we were unable to ascertain whether the virus had a resistance mutation. However, we observed that the initial BALF specimens were continuously positive for avian influenza A (H7N9) until 22 days after symptom onset. The BALF specimen was negative for influenza A (N9) virus on the 25th day after symptom onset, whereas the specimen remained positive for influenza A (H7) virus at that time. Finally, the BALF specimens were tested again on the 28th and 29th days after symptom onset and were negative for influenza A (H7N9) virus by RT-PCR. These results suggested a decreasing trend in the viral load during the oseltamivir treatment, although the BALF RT-PCR tests conducted were not quantitative assays. Furthermore, a previous study has reported that prolonged viral shedding may occur in patients with severe avian influenza A (H7N9) despite oseltamivir treatment (1), and that this might occur for up to 20 days (8), which is similar to our findings. Thus, we believe that oseltamivir treatment should have been effective in our patient. However, the resulting continuous viral replication significantly prolonged the course of the disease; moreover, an extended course of virus infection may contribute to continuous alveolar damage and severe interstitial fibrosis, as seen in our patient, which may greatly increase the risk of death from serious H7N9 virus infection. During the late course of the disease, the patient’s clinical status continuously deteriorated with a progressive decline in lung compliance and the development of refractory hypoxemia. However, laboratory examinations showed that the serum procalcitonin levels and white blood cell counts were normal. Concurrently, repeat chest X-rays revealed that the initial large infiltrates and consolidation shadows had clearly resolved in both lungs. Moreover, the final histological changes of the lung sections obtained from the limited autopsy after the patient’s death showed numerous lymphocytic infiltrates but only few neutrophilic infiltrates without lung abscesses, which suggest no obvious evidence of severe pulmonary co-infection. The final histological findings also showed diffuse severe pulmonary fibrosis, which is compatible with the clinical manifestations. The development of multi-organ failure in this patient was considered to be due to refractory hypoxemia as a result of severe pulmonary fibrosis. Finally, he died after rapid deterioration 39 days after symptom onset, despite corticosteroid treatment.
In conclusion, a lengthy course of avian influenza A (H7N9) virus infection may contribute to severe alveolar damage and interstitial fibrosis, which may result in a high mortality rate. During the late course of viral infection, various pathological changes and pulmonary damage, particularly severe interstitial fibrosis, may be observed, which suggest the beginning of the fibroproliferative phase. Unfortunately, a full patient autopsy could not be obtained, because of religious reasons. More studies are needed to expand our understanding of the disease pathogenesis, to improve the treatment success rate and to reduce the complications and mortality rate due to severe H7N9 infection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yu L, Wang Z, Chen Y, et al. Clinical, virological, and histopathological manifestations of fatal human infections by avian influenza A(H7N9) virus. Clin Infect Dis 2013;57:1449-57. [PubMed]
- Nicholls JM, Tsai PN, Chan RW, et al. Fatal H7N9 pneumonia complicated by viral infection of a prosthetic cardiac valve - an autopsy study. J Clin Virol 2014;61:466-9. [PubMed]
- Feng Y, Hu L, Lu S, et al. Molecular pathology analyses of two fatal human infections of avian influenza A(H7N9) virus. J Clin Pathol 2015;68:57-63. [PubMed]
- Guo Q, Huang JA, Zhao D, et al. Pathological changes in a patient with acute respiratory distress syndrome and H7N9 influenza virus infection. Crit Care 2014;18:666. [PubMed]
- He YX, Gao ZF, Lu M, et al. A histopathological study on Influenza A H1N1 infection in humans. Beijing Da Xue Xue Bao 2010;42:137-9. [PubMed]
- Bai YQ, Xu G, Gong ZL, et al. Pathologic changes caused by highly pathogenic H5N1 avian influenza virus: postmortem study of a case. Zhonghua Bing Li Xue Za Zhi 2006;35:545-8. [PubMed]
- Lu M, Xie ZG, Gao ZC, et al. Histopathologic study of avian influenza H5N1 infection in humans. Zhonghua Bing Li Xue Za Zhi 2008;37:145-9. [PubMed]
- Chen Y, Liang W, Yang S, et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet 2013;381:1916-25. [PubMed]