Precise delineation of clinical target volume for crossing-segments thoracic esophageal squamous cell carcinoma based on the pattern of lymph node metastases
Introduction
Despite multimodality therapy, the prognosis of esophageal squamous cell carcinoma (ESCC) remains dismal, with overall 5-year survival rate for patients with lymph node metastasis (LNM) after surgical resection only 20% (1). LNM was one of the most important factors in predicting the prognosis of patients (2,3).
In 2010, the present (7th) edition of the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC) TNM staging system for ESCC was released (4). This edition redefined tumor location in details. Previously, plenty of studies had revealed that different tumor location was characterized by different pattern of LNM, for example, upper thoracic tumor drained most into the upper mediastinal, middle thoracic tumor was characterized with skip metastasis, whereas lower thoracic tumor tended to metastasize to abdominal lymph nodes (5). Embryological difference may account for different lymphatic flow among esophagus segments (6,7).
Theoretically, radiation oncologists delineated clinical target volume (CTV) mainly according to patients’ tumor location and imaging tests, and then the first step of radiotherapy contouring was finished. However, in fact, it was not that easy. First of all, opinions on boundary of lymph node station on the computed tomography (CT) images did not reach consensus among radiation oncologists. Hopefully, our recently published article may improve the situation (8). Secondly, crossing-segments thoracic ESCC was often encountered in clinical practice. And this could lead to sort of subjective identification of tumor location, which was usually stated such as ‘determined by the location of the main lesion’ (9). This brought inconsistency to CTV delineation and troubled scientific researches. Thus, the present study was conducted to explore LNM pattern of crossing-segments thoracic ESCC and to give recommendation on precise delineation of CTV.
Materials and methods
Patient population
During the period between January 2000 and December 2014, a retrospective analysis was conducted on 3,587 patients with thoracic ESCC who underwent surgery at Shandong Cancer Hospital and Institute (Jinan, China). Informed consent was obtained from patients prior to surgery. The inclusion criteria were as follows: (I) pathologically confirmed thoracic ESCC with single lesion; (II) patients with preoperative work-up including cervical ultrasonography and endoscopic ultrasonography (EUS), high-resolution CT; (III) neither chemotherapy or radiotherapy given prior to esophagectomy; and (IV) an extended esophagectomy with surgery performed. The following patients were excluded: (I) patients with distant metastasis; (II) patients who underwent surgery without curative intent; (III) patients with mixed histological types; (IV) other surgery procedure instead of extended esophagectomy with three-field approach (3-FL) or two-field approach (2-FL) performed; and (V) patients with other tumors at the time of diagnosis.
The definition of ESCC location
According to 7th AJCC, typical endoscopic measurements for the upper thoracic esophagus measured from the incisors are from 20 to <25 cm, middle thoracic esophagus from 25 to <30 cm, lower thoracic esophagus from 30 to 40 cm. In the present study, the location of the primary tumor was defined by the position of the cancer center which was calculated by the tumor’s upper and lower end of endoscopic measurements. When esophagus was too narrow for the probe of EUS to get through, upper end measured from the incisors and tumor length from CT report was used to determine tumor center. When the upper and lower ends were both within middle thoracic esophagus, it was defined as middle thoracic ESCC. When tumor center was within the middle thoracic esophagus but the upper ends extended to upper thoracic esophagus, it was defined as middle-upper thoracic ESCC. Similarly, tumor with center within upper thoracic esophagus and lower end extending to middle thoracic esophagus was defined as upper-middle thoracic ESCC. For middle thoracic tumors extending both upwards to upper thoracic esophagus and downwards to lower thoracic tumor, it was defined as upper-middle-lower thoracic ESCC.
Surgical procedures
Patients without cervical lymph node swelling (short diameter less than 5 mm) by preoperative work-up received 2-FL lymph node dissection including total mediastinal, perigastric, and celiac lymph node. If enlarged lymph nodes (short diameter greater than 5 mm) were detected, cervical dissection was added to make 3-FL lymphadenectomy which was performed through a right thoracotomy, laparotomy, and bilateral cervical collar. For lesions in the upper third of the thoracic segment, esophageal resection was performed via right thoracotomy using a stomach for esophageal replacement. For lesions in the middle and lower third, esophagectomy was performed on the left side using the stomach to establish digestive continuation. Great care was taken not to damage the recurrent laryngeal nerves, superior vena cava, innominate artery, right subclavian artery and pulmonary branch of the right vagal nerve.
Lymph node mapping
Lymphatic nodes were named according to the guideline of the Japanese Society for Esophageal Diseases (JSED) (Table 1) (10,11). If lymph node station was not recorded as clearly as JSED described, two thoracic surgeons (Zhao and Liu) and three experienced radiation oncologists (Huang, Zhang, Zhou) were engaged jointly to define the extent of lymph node dissection, number of node in each compartment (upper, middle, lower mediastinum and abdomen) and exact lymph node station.
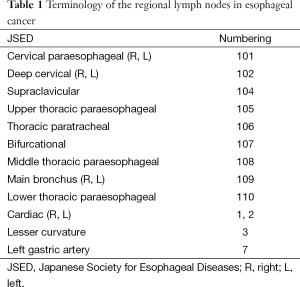
Full table
Results
Patients and clinicopathologic features
The study included 3,587 patients with thoracic ESCC and a median age of 61-year who underwent esophagectomy. Mean tumor length was 4.7 cm. Pathologic morphology type was 1,761 (49.1%), 1,032 (28.8%), 539 (15.0%), 252 (7.0%), 3 (0.1%) for ulcering, medullary, fungating, narrow and intracavity type respectively. In terms of pathological stage, it was 435 (12.1%), 935 (26.1%), 1,992 (55.5%), 225 (6.3%) for pT1, pT2, pT3 and pT4. 2,223 (62.0%), 1,233 (34.1%), 98 (2.7%), 33 (1.0%) for pN0, pN1, pN2, pN3. A total of 1,145 (31.9%) patients were well differentiated, 1,622 (45.2%) patients were moderately differentiated, and 820 (22.9%) were poorly differentiated. A total of 3,115 (86.8%) patients received 2-FL surgery, whereas 472 (13.2%) patients underwent 3-FL surgery. More details were listed in Table 2.
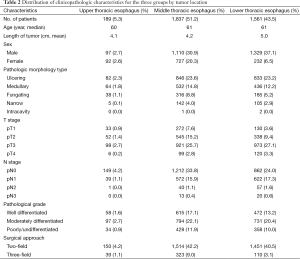
Full table
Tumor location and LNM
Among the patients reviewed, 189 (5.3%), 1,837 (51.2%) and 1,561 (43.5%) were in the upper, middle and lower thoracic esophagus, respectively. A total of 1,501 (41.8%) was crossing-segments thoracic ESCC patients.
A total of 71,740 nodes had been removed with a mean of 20 (range, 16–50) nodes per patient. The incidence of LNM was 4.0% (2,870/71,740), the ratio of positive lymph node to lymph nodes resected. About 38.0% (1,364/3,587) patients suffered lymphatic metastases. Moreover, the rate of LNM was defined as numbers of positive lymph node patients to relative total patients. They were 12.1%, 15.2%, 8.0%, 3.0%, and 7.1% in neck, upper mediastinum, middle mediastinum, lower mediastinum, and abdominal cavity for patients with upper-middle thoracic ESCC, 10.3%, 8.2%, 11.0%, 4.8%, 8.2% for middle-upper thoracic ESCC, 4.8%, 4.8%, 24.1%, 6.3%, 22.8% for middle-lower thoracic ESCC and 3.9%, 3.1%, 22.8%, 11.9%, 25.8% for lower-middle thoracic ESCC, respectively (Table 3). Data of eight patients with upper-middle-lower thoracic ESCC was not demonstrated in Table 3.
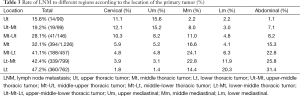
Full table
Table 4 showed the sequence of LNM to different regions according to primary tumor location. The top three sites of LNM were 105 (12.1%), 108 (6.0%), 101 (6.1%) for upper-middle thoracic ESCC, 108 (8.2%), 105 (7.5%), 106 (6.8%) for middle-upper thoracic ESCC, 1 (18.8%), 108 (17.9%), 107 (9.6%) for middle-lower thoracic ESCC, 1 (21.3%), 108 (16.1%), 107 (10.1%) for lower-middle thoracic ESCC.
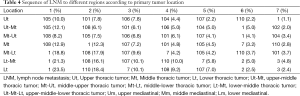
Full table
Discussion
Radiotherapy plays a significant role in ESCC, especially for those who could not undergo surgery. Design of irradiation field was critical to improve efficacy while minimizing toxicities. In clinical practice, CTV delineation was commonly based on tumor location (12). For tumors entirely located within the esophagus anatomic segments, tumor location could be easily recognized and then CTV could be delineated accordingly. However, doctors often encounter patients with tumors crossing the adjacent anatomic segments. In that case, inconsistency in defining CTV may inevitably occur among radiation oncologists. This inconsistency may offset the benefits of 3D treatment planning with high-precision dose delivery (13). Thus, to minimize inconsistency of irradiation field and to achieve precise radiotherapy, we investigated LNM pattern of crossing-segments thoracic ESCC.
The seventh edition of the AJCC Cancer Staging Manual redefined tumor location as the length between the upper incisor and the upper edge of the tumor, not the tumor center any more. We speculated that this change was made mainly by taking esophageal adenocarcinoma prognosis and surgical indications into consideration rather than ESCC and radiotherapy. From the perspective of ESCC and radiotherapy, we doubted the biological significance of choosing upper edge to define tumor location, because empirically tumor cells invaded not only downwards but also upwards. Thus, we chose tumor center to describe tumor location.
According to our results, crossing-segments thoracic ESCC accounted for 41.8% of the total patients. Previous studies didn’t specifically address this subpopulation (14,15). Table 3 revealed some interesting results. Firstly, middle-upper thoracic ESCC was commonly clarified as middle thoracic ESCC by previous definition. However, its LNM pattern was to some extent distinct from middle thoracic ESCC and middle-lower thoracic ESCC, because its extrathoracic LNM was much smaller (8.2%). Secondly, middle mediastinal LNM of middle-lower thoracic ESCC exceeds considerably that of middle thoracic ESCC (24.1% vs. 16.6%). Similarly, lower mediastinal LNM of lower-middle thoracic ESCC was remarkably higher than that of middle-lower thoracic ESCC (11.9% vs. 6.3%). This indicated that the location of tumor center reflected LNM pattern to some extent: tumors with lower location tend to metastasize to lower sites.
Due to the different types of esophagus cancer between western and eastern countries, elective nodal irradiation (ENI) including high-risk lymph nodal regions is often performed in East-Asia regions (16). However, design of CTV has not reached consensus yet. RTOG 85-01 adopted target delineation of the supraclavicular fossae to the esophagogastric junction for thoracic esophagus cancer (17). National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN) recommended to design irradiation field based on primary tumor location: for proximal third of esophagus, irradiation field included para-esophageal lymph node and supraclavicular lymph nodes; for middle third of esophagus, para-esophageal lymph nodes were irradiated without treatment of abdominal lymph nodes; in terms of distal third esophagus, para-esophageal lymph nodes and abdominal lymph nodes were incorporated into irradiation field. However, which lymph node sites should be covered was not specifically elucidated in NCCN. Nakamura et al. investigated irradiation field based on the postoperative pathological results (9). It was reported that for upper thoracic esophagus, superior border of irradiation field should be 101 and 104, meanwhile inferior border of irradiation field should be 109. For middle thoracic esophagus, superior border of irradiation field should be 101 and 106, inferior border of irradiation field should be 3. For lower thoracic esophagus, superior border of irradiation field should be 110, inferior border of irradiation field should be 3. Although Nakamura et al. gave specific advisement on lymph node sites delineation, it did not explore LNM pattern of crossing-segments thoracic ESCC and the sample number was relatively small.
Based on clinical experiences from our institution and results of the present study (8,14,15), we suggested the following methods of target delineation for crossing-segments thoracic ESCC (Figure 1). For upper-middle thoracic ESCC, delineation of CTVn should include 101, 104, 105, 106, 107, part of 108. As was well-known, irradiation of abdominal lymph nodes was expected to be carefully decided, for it may significantly influence patients’ life quality. Considering lower abdominal LNM rate of middle-upper thoracic ESCC (8.2%), we suggested that middle-upper should be free from abdominal lymph nodes irradiation. CTVn of 101, 104, 105, 106, 107, 108, part of 110 was recommended for middle-upper thoracic ESCC. Whereas, for middle-lower thoracic ESCC, abdominal lymph nodes irradiation was expected to be added to CTVn with abdominal cavity LNM of 22.8%. In addition, the superior margin of CTVn should extend to 105. For lower-middle thoracic ESCC, we advocated the inferior margin should include 1, 2, 3, 7 and the superior margin of CTVn extended to 107, while 105 and 106 were not necessary to be included, because LNM of 105 and 106 were only 2.1% and 1.8% (not shown in the table), respectively.
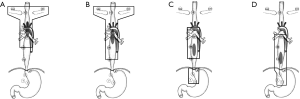
We have to point out that preoperative work up played a significant role in treatment for patients who refuse operation or unsuitable to operation. EUS is a widely used technique for T and N staging of esophageal cancer. Rösch et al. reported accuracy of T staging as 89% (18). For the N stage, especially with the use of fine needle aspiration (FNA), the diagnostic LNM accuracy of EUS reached 87–100% (19). CT was also applied to assess N stage and distant metastases (20). The sensitivity of chest CT in detecting distant metastases of nodules 10 mm or larger reached about 90% (21). PET/CT facilitated more sensitive detection of distant metastases (22). The development of multimodality assessment is making preoperative work up more and more consistent with postoperative results (the gold standard). We believed that patients who could not undergo surgery could refer to our suggestions. In addition, for lesions in the lower third of the thoracic segment and patients aged over 70, 3-FL lymphadenectomy was not routinely performed in our institution. Instead, we preformed cervical ultrasonography before operation. If cervical lymph node was reported swelling and patients were expected to tolerate the surgery, 3-FL was performed by experienced surgeons. This lead to 13.2% 3-FL. Considering treatment-related toxicities, we thought blind pursuit of large irradiation field covering every minor LNM site was unacceptable. Moreover, chemotherapy played significant role in systematic therapy. So, we suggested the irradiation field in the present study be adopted in definite chemoradiotherapy.
It was noteworthy that a related phase III study based on our present and previous studies is ongoing in China to compare efficacy of ENI and involved field irradiation received by patients with ESCC. Future data including recurrence pattern, treatment-related toxicities and survival will help to improve limitations of this article and update our knowledge.
Conclusions
Radiotherapy for patients with crossing-segments thoracic ESCC was supposed to be individualized. The lymph nodes included in the irradiation field should be determined according to the primary site in the esophagus. For upper-middle and middle-upper thoracic ESCC, abdominal cavity may be free from irradiation and inferior margin of irradiation field should be appropriately extended for middle-upper thoracic ESCC. For middle-lower and lower-middle thoracic ESCC, their LNM pattern resembled each other. Moreover, irradiation of abdominal cavity can’t be neglected, and superior margin of irradiation field should be adjusted accordingly for middle-lower thoracic ESCC.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (81201526) and Shandong Province Natural Science Foundation (ZR2012HQ009).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen W, He Y, Zheng R, et al. Esophageal cancer incidence and mortality in China, 2009. J Thorac Dis 2013;5:19-26. [PubMed]
- Baba M, Aikou T, Yoshinaka H, et al. Long-term results of subtotal esophagectomy with three-field lymphadenectomy for carcinoma of the thoracic esophagus. Ann Surg 1994;219:310-6. [PubMed]
- Mariette C, Piessen G, Briez N, et al. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg 2008;247:365-71. [PubMed]
- Edge S, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. New York: Springer-Verlag. 2010.
- Chen J, Liu S, Pan J, et al. The pattern and prevalence of lymphatic spread in thoracic oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg 2009;36:480-6. [PubMed]
- Skandalakis JE, Gray SW. Embryology for surgeons: the embryological basis for the treatment of congenital anomalies. Baltimore: Williams & Wilkins. 1994.
- Skinner DB. En bloc resection for neoplasms of the esophagus and cardia. J Thorac Cardiovasc Surg 1983;85:59-71. [PubMed]
- Huang W, Huang Y, Sun J, et al. Atlas of the thoracic lymph nodal delineation and recommendations for lymph nodal CTV of esophageal squamous cell cancer in radiation therapy from China. Radiother Oncol 2015;116:100-6. [PubMed]
- Nakamura T, Hatooka S, Kodaira T, et al. Determination of the irradiation field for clinical T1-T3N0M0 thoracic/abdominal esophageal cancer based on the postoperative pathological results. Jpn J Clin Oncol 2009;39:86-91. [PubMed]
- Fujita H, Sueyoshi S, Tanaka T, et al. Three-field dissection for squamous cell carcinoma in the thoracic esophagus. Ann Thorac Cardiovasc Surg 2002;8:328-35. [PubMed]
- Japanese Society for Esophageal Diseases. Guide lines for the clinical and pathologic studies for carcinoma of the esophagus. Jpn J Surg 1976;6:79-86. [PubMed]
- Yamashita H, Okuma K, Wakui R, et al. Details of recurrence sites after elective nodal irradiation (ENI) using 3D-conformal radiotherapy (3D-CRT) combined with chemotherapy for thoracic esophageal squamous cell carcinoma--a retrospective analysis. Radiother Oncol 2011;98:255-60. [PubMed]
- Tai P, Van Dyk J, Yu E, et al. Variability of target volume delineation in cervical esophageal cancer. Int J Radiat Oncol Biol Phys 1998;42:277-88. [PubMed]
- Cheng J, Kong L, Huang W, et al. Explore the radiotherapeutic clinical target volume delineation for thoracic esophageal squamous cell carcinoma from the pattern of lymphatic metastases. J Thorac Oncol 2013;8:359-65. [PubMed]
- Huang W, Li B, Gong H, et al. Pattern of lymph node metastases and its implication in radiotherapeutic clinical target volume in patients with thoracic esophageal squamous cell carcinoma: A report of 1077 cases. Radiother Oncol 2010;95:229-33. [PubMed]
- Onozawa M, Nihei K, Ishikura S, et al. Elective nodal irradiation (ENI) in definitive chemoradiotherapy (CRT) for squamous cell carcinoma of the thoracic esophagus. Radiother Oncol 2009;92:266-9. [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [PubMed]
- Rösch T. Endosonographic staging of esophageal cancer: a review of literature results. Gastrointest Endosc Clin N Am 1995;5:537-47. [PubMed]
- Plukker JT, van Westreenen HL. Staging in oesophageal cancer. Best Pract Res Clin Gastroenterol 2006;20:877-91. [PubMed]
- Sgourakis G, Gockel I, Lyros O, et al. Detection of lymph node metastases in esophageal cancer. Expert Rev Anticancer Ther 2011;11:601-12. [PubMed]
- Becker CD, Barbier PA, Terrier F, et al. Patterns of recurrence of esophageal carcinoma after transhiatal esophagectomy and gastric interposition. AJR Am J Roentgenol 1987;148:273-7. [PubMed]
- Luketich JD, Friedman DM, Weigel TL, et al. Evaluation of distant metastases in esophageal cancer: 100 consecutive positron emission tomography scans. Ann Thorac Surg 1999;68:1133-6; discussion 1136-7. [PubMed]


