Immune checkpoint inhibitors in neoadjuvant therapy of non-small cell lung cancer: a systematic review and meta-analysis
Introduction
Lung cancer currently causes the highest morbidity and mortality worldwide. According to 2020 cancer statistics, there were 220,000 new lung cancer cases and approximately 13,500 deaths in the United States (1). Radical surgery is the mainstay of treatment for early-stage non-small cell lung cancer (NSCLC) (I–IIIA) but there is still a higher risk of long-term recurrence and distant metastasis (2). Therefore, systemic treatment plays an important role in improving the prognosis of lung cancer patients (3).
Systemic treatment of lung cancer includes adjuvant chemotherapy and neoadjuvant chemotherapy as recommended by National Comprehensive Cancer Network (NCCN) guidelines (4). In recent years, inhibitors of programmed cell death receptor-1 (PD-1) and programmed death-ligand 1 (PD-L1) have shown good efficacy in the treatment of advanced NSCLC. Studies had shown that the 5-year overall survival of patients with advanced NSCLC who received immune checkpoint inhibitor therapy is 15–16% (5,6).
Although it is not a part of the standard treatment currently, anti-PD-1/PD-L1 therapy has already been evaluated in clinical trials, due to its proven efficacy in advanced stages and its low toxicity. In 2020, Provencio et al. reported an 83% major pathological remission (MPR) rate when nivolumab was used for neoadjuvant treatment of resectable NSCLC (7). However, another study reported that the neoadjuvant treatment regimen that combines chemotherapy and anti-PD-1 resulted in only 27% of MPRs (8). The effect of neoadjuvant ICIs in neoadjuvant therapy of NSCLC is contradictory, therefore, a systematic review and evaluation of neoadjuvant treatments for ICIs are needed. To date, there is only one systematic review showing that ICI therapy may be a viable treatment option for NSCLC patients before surgery. However, since most of the information came from conference abstracts and lacks statistical differences, further work is still needed. Therefore, we conducted a meta-analysis to evaluate the feasibility and safety of ICIs for the neoadjuvant therapy of NSCLC.
We present the following article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1664/rc).
Methods
Literature search
Embase, PubMed and Web of Science were systematically searched from 1st January 2018 to 1st August 2021 to include proper studies with a search query combining synonyms of lung cancer, neoadjuvant and ICIs as follows: (lung OR non-small cell lung cancer OR squamous OR adenocarcinoma) AND (cancer OR carcinoma OR tumour OR neoplasm) AND (neoadjuvant OR preoperative OR perioperative) AND (ICIs OR “immune checkpoints”). We also searched the “clinicaltrials.gov” site to identify related clinical trials. The articles cited in the papers were screened to increase the quantity of relevant literature.
Study selection
Studies fulfilling the following criteria were included in this meta-analysis: (I) studies with at least one treatment with an ICI were eligible for inclusion; (II) the target population was untreated/ICI-naive potentially resectable NSCLC patients; (III) at least one of the efficacy results expressed as objective response rate (ORR), MPR, or pathologic complete remission (pCR); (IV) feasibility data including treatment-related adverse reaction (TRAE), immune-related adverse event (irAE) and surgery-related complications had to be provided.
Studies were excluded if one of the following existed: (I) other publications apart from original articles (such as review articles, letters, conference abstracts and editorials); (II) studies focused on other subjects (e.g., the treatment regimen is neoadjuvant immunotherapy but provides information other than safety and feasibility); (III) studies not using ICIs; (IV) studies with overlapping patient populations; (V) studies that did not provide sufficient information to evaluate the safety and feasibility of NSCLC neoadjuvant immunotherapy.
The literature search and study selection were performed by two reviewers, with a third independent reviewer consulted to reach a consensus in the event of any disagreements.
Data extraction and quality assessment
Raw data were extracted from the selected studies by two reviewers and checked for consensus. When studies adopted different ICIs schemes but no separate data was provided, pooled results were used and when more than two types of ICIs were used, the data were included in the ICIs subgroup. Data were extracted using a standard form: (I) study characteristics: study origin (authors and years), study design (NCT number and study phase); (II) patient characteristics: study population, medium age and range, percentage of males and smoking history; (III) tumour characteristics: tumour stages and percentage of patients with ≥ N2 lymph node metastasis; (IV) neoadjuvant characteristics: the specific name of the ICI, neoadjuvant regimen and neoadjuvant cycles.
The quality of the selected studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool (9). Quality control and consensus view were also performed by two independent reviewers.
Data synthesis and analysis
All the included studies were single-arm studies and mainly compare the rate of MPR, ORR, pCR, etc., the included data were transformed according to the following formula because they were not normally distributed.
Where X is the number of occurrences of an event, n is the total number of observed patients. The Higgins I2 test with inconsistency index was used to assess heterogeneity as follows: 0–40% heterogeneity might not be important; 30–60% moderate heterogeneity; 50–90% substantial heterogeneity and 75–100% considerable heterogeneity (10). Review Manager 5.3 (Cochrane) was used for statistical analyses with a P value of <0.05 indicating statistical significance. The odds ratio (OR) value and 95% confidence interval (CI) were transformed to obtain the final effect index:
Where Pf: rate of the final effect; LL: the lower limit of the 95% CI for the final effect; LLOR: the lower limit of the 95% CI for the OR; UL: the higher limit of the 95% CI for the final effect; ULOR: the higher limit of the 95% CI for the OR.
Results
Literature search
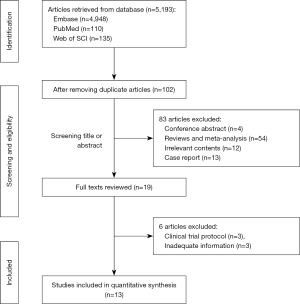
The systematic literature search initially retrieved 5,193 articles and after duplicates were removed, the titles and abstracts of the 102 remaining articles were screened, identifying 19 potential articles. The full-text articles were reviewed and thirteen studies involving 358 patients met the inclusion criteria (7,8,11-21) (Figure 1), with six studies excluded for the following reasons: clinical trial protocol (n=3), inadequate information (n=3).
Study characteristics
The study characteristics are shown in Table 1. There were five retrospective studies and eight prospective studies. Amongst the study population, the medium age ranged from 61 to 67 years, with 47–85% male patients and the smoking history ranged from 73% to 100%. Tumour stages ranged from Ia to IVb, and seven studies reported ≥ N2 lymph metastasis. Different ICIs, including anti-PD-1 drugs like nivolumab, pembrolizumab, sintilimab, toripalimab and anti-PD-L1 drugs like atezolizumab, durvalumab and avelumab were utilised in the different studies. The neoadjuvant treatment options in seven studies combined both ICIs and chemotherapy, and the neoadjuvant cycles were between 2 to 6.
Table 1
| Studies | Study characteristics | Patient characteristics | Tumor characteristics | Neoadjuvant characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | NCT number | Phase | Medium age [range] (years) | Male, n [%] | Smoking history, n [%] | Stage | ≥ N2, n [%] | ICI regimen | Neoadjuvant regimen | Neoadjuvant cycle | ||||
| Bott | 2019 | NCT02259621 | I | 67 [55–84] | 10 [48] | 18 [86] | I–IIIa | 4 [20] | Nivolumab | ICI | 2 | |||
| Cascone | 2021 | NCT03158129 | II | 66 [±8.3] | 28 [64] | 36 [82] | Ia–IIIa | 7 [16] | Nivolumab+ ipilimumab | ICI | 3 | |||
| Chen T | 2021 | NR | NR | 61 [55–67] | 9 [75] | 9 [75] | IIIa–IIIb | NR | Nivolumab/pembrolizumab | Chem + ICI | 2/4 | |||
| Chen Y | 2021 | NR | NR | 62 [43–72] | 29 [83] | NR | IIIa–IIIb | 19 [54] | Pembrolizumab | Chem + ICI | 2 | |||
| Forde | 2018 | NCT02259621 | NR | 67 [55–84] | 10 [48] | 18 [86] | I–IIIa | NR | Nivolumab | ICI | 2 | |||
| Gao | 2020 | CHICTR-OIC-17013726 | Ib | 62 [47–70] | 33 [83] | 32 [80] | Ia–IIIb | NR | Sintilimab | ICI | 2 | |||
| Liu | 2020 | NR | NR | 63 [NR] | 11 [85] | 11 [85] | II–III | NR | Pembrolizumab/toripalimab | Chem + ICI | 2 | |||
| Lücke | 2020 | NR | NR | 67 [55–78] | 2 [50] | NR | IIIb–IVb | 3 [75] | Pembrolizumab/atezolizumab | ICI | 2-6 | |||
| Provencio | 2020 | NCT03081689 | II | 63 [58–70] | 34 [74] | 46 [100] | IIIa | 34 [74] | Nivolumab | Chem + ICI | 3 | |||
| Reuss | 2020 | NCT02259621 | Ib/II | 63 [48–78) | 7 [78] | 9 [100] | Ib–IIIa | NR | Nivolumab + ipilimumab | ICI | 3 | |||
| Shu | 2020 | NCT02716038 | II | 67 [62–74] | 15 [50] | NR | Ib–IIIa | 19 [63] | Atezolizumab | Chem + ICI | 3 | |||
| Tfayli | 2020 | NR | NR | 65 [45–80] | 7 [47] | 11 [73] | Ib–IIIa | NR | Avelumab | Chem + ICI | 4 | |||
| Rothschild | 2021 | NCT02572843 | II | 61 [41–74] | 35 [53] | 64 [95.5] | IIIa | 67 [100] | Durvalumab | Chem + ICI | 3 | |||
NR, not related; ICI, immune checkpoint inhibitor; Chem, chemotherapy; NR, not related.
Safety
Table 2 shows the perioperative adverse events reported in the studies. The number of patients ranged from 4 to 67, with 218 of the 358 patients receiving ICI and chemotherapy-containing regimens (Table 3) and the other 140 patients included in six studies receiving ICIs only. The 157 [157/218 (72.0%)] patients who received combined neoadjuvant therapy showed a higher incidence of TRAEs, while only 37 (26.4%) patients who received neoadjuvant ICIs only experienced TRAEs. During neoadjuvant immunotherapy, TRAEs ranged from 0 to 100%, while ≥ grade 3 irAEs ranged from 0 to 88.1%. Grade 3 or higher irAEs were observed in 92 of 358 (25.7%) patients (7,8,11,12,14-16,19-21). Among the 92 patients, the rate of ≥ grade 3 irAEs [81/218 (37.2%)] was observed in the neoadjuvant immunochemotherapy subgroup (7,8,14,20,21) than neoadjuvant ICIs group [11/140 (7.9%)] (11,12,15,16,19).
Table 2
| Studies | Year | No. of patients | TRAEs, n (%) | 3–4 irAEs, n (%) | Resectable patients, n (%) | Surgery delay, n (%) | Complications, n (%) | MPR, n (%) | pCR, n (%) | ORR, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bott | 2019 | 22 | 2 (9.1) | 1 (4.5) | 20 (90.9) | 0 (0) | 10 (50.0) | 9 (45.0) | NR | 2 (9.1) |
| Cascone | 2021 | 44 | 3 (6.8) | 2 (4.5) | 39 (88.6) | NR | NR | 13 (33.3) | 8 (20.5) | 9 (20.5) |
| Chen T | 2021 | 12 | 4 (33.3) | 0 (0) | 12 (100.0) | 0 (0) | 3 (25.0) | 9 (75.0) | 5 (41.7) | 6 (50.0) |
| Chen Y | 2021 | 35 | 1 (2.9) | 1 (2.9) | 35 (100.0) | 0 (0) | 0 (0) | 26 (74.3) | 18 (51.4) | 17 (48.6) |
| Forde | 2018 | 21 | 5 (23.8) | 1 (4.8) | 20 (95.2) | 0 (0) | NR | 9 (45.0) | 3 (15.0) | 2 (9.5) |
| Gao | 2020 | 40 | 21 (52.5) | 4 (10.0) | 37 (92.5) | 2 (5.4) | 4 (10.8) | 15 (40.5) | 9 (24.3) | 8 (21.6) |
| Liu | 2020 | 13 | 12 (92.3) | 0 (0) | 5 (38.5) | 0 (0) | 5 (100.0) | 3 (60.0) | 1 (20.0) | 8 (61.5) |
| Lücke | 2020 | 4 | 0 (0) | 0 (0) | 4 (100.0) | 0 (0) | 0 (0) | 2 (50.0) | 2 (50.0) | 3 (75.0) |
| Provencio | 2020 | 46 | 43 (93.5) | 14 (30.4) | 41 (89.1) | 0 (0) | 12 (29.3) | 34 (82.9) | 26 (63.4) | 35 (76.1) |
| Reuss | 2020 | 9 | 6 (66.7) | 3 (33.3) | 6 (66.7) | 0 (0) | 1 (16.7) | 2 (33.3) | 2 (33.3) | 1 (16.7) |
| Shu | 2020 | 30 | 26 (86.7) | 3 (10.0) | 26 (86.7) | 0 (0) | 13 (50.0) | 17 (65.4) | 10 (38.5) | 19 (63.3) |
| Tfayli | 2020 | 15 | 4 (26.7) | 4 (26.7) | 11 (73.3) | 0 (0) | 0 (0) | 3 (27.3) | 1 (9.1) | 4 (26.7) |
| Rothschild | 2021 | 67 | 67 (100.0) | 59 (88.1) | 55 (82.1) | NR | 2 (3.6) | 34 (61.8) | 10 (18.2) | 29 (43.3) |
NSCLC, non-small-cell lung cancer; No., number; TRAEs, treatment-related adverse events; irAEs, immune-related adverse events; MPR, major pathologic response; pCR, pathological complete response; ORR, objective response rate; NR, not related.
Table 3
| Neoadjuvant regimen | No. of patients | TRAEs, n (%) | 3-4 irAEs, n (%) | Resectable patients, n (%) | Surgery delay, n (%) | Complications, n (%) | MPR, n (%) | pCR, n (%) | ORR, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| ICI | 140 | 37 (26.4) | 11 (7.9) | 126 (90.0) | 2 (1.6) | 15 (11.9) | 50 (39.7) | 24 (19.0) | 25 (17.8) |
| Chem + ICI | 218 | 157 (72.0) | 81 (37.2) | 185 (84.9) | 0 (0) | 35 (18.9) | 126 (68.1) | 71 (38.4) | 118 (54.1) |
NSCLC, non-small-cell lung cancer; No., number of; TRAEs, treatment-related adverse events; irAEs, immune-related adverse events; MPR, major pathologic response; pCR, pathological complete response; ORR, objective response rate; ICI, immune checkpoint inhibitor; Chem, chemotherapy.
The surgical resection rate was between 38.5–100%, with only two patients experiencing a delay in surgery (16). Complication rates ranged between 16.7–100% among the eight studies that reported postoperative complications. More postoperative complications [35/185 (18.9%)] were identified in the neoadjuvant immunochemotherapy subgroup, while there was no direct evidence that this was related to the combined application of ICIs and chemotherapy.
Feasibility
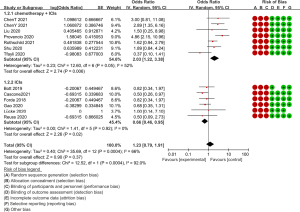
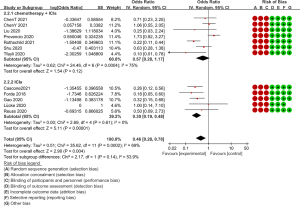
All thirteen studies provided pathological efficacy evaluation data, indicating the presence of less than 10% residual viable tumour cells in the resected primary tumour. Of the 176 people who achieved an MPR, 126 received ICI and chemotherapy combined neoadjuvant therapy. The MPR rates of each study ranged from 27.3% to 82.9%. Of all the seven studies on neoadjuvant immunochemotherapy (7,8,13,14,17,20,21), the MPR rates were all higher than 60% except for Tfayli et al. (8). Meanwhile, compared to the neoadjuvant immunotherapy group, the OR value of the MPR rate in the neoadjuvant immunochemotherapy group was significantly higher (OR =0.55, 95% CI: 0.44–0.66, P=0.0004, after transformation) (Figure 2). The pCR is another useful tool for predicting the efficacy of neoadjuvant therapy, with all but one study providing pCR data (11), with the pCR ranging between 9.1–63.4%. Seventy-one of the 95 patients who achieved pCR had undergone ICI and chemotherapy. Consistent with this finding, the OR value of the pCR rate in the neoadjuvant immunochemotherapy group was higher (OR =0.32, 95% CI: 0.22–0.44, P=0.14, after transformation) (Figure 3). Although the P value (0.14) >0.05, there are differences in trends and the Higgins I2 is 53.9%, which suggests that there is moderate heterogeneity between the two subgroups.
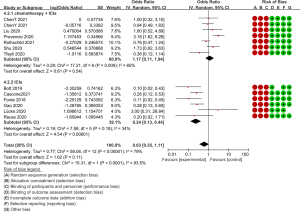
Another important piece of evidence to measure feasibility is the ORR based on the RECIST 1.1 criteria (22). Patients who achieved partial response (PR) and complete response (CR) were considered radiological responders and the ORR ranged between 9.1% and 75%. Patients undergoing ICI and chemotherapy achieved more radiological response [118/218 (54.1%)] than patients undergoing ICIs [25/140 (17.9%)] only, with the OR value of the ORR also showing a significant difference between the two groups (OR =0.39, 95% CI: 0.26–0.53, P<0.0001, after transformation) (Figure 4).
Discussion
Based on the previous excellent performance of ICIs in the treatment of advanced lung cancer (5,6), the application of ICIs in neoadjuvant treatment of NSCLC is currently being explored. For the exploration of perioperative immunotherapy, the results of a phase IB study and a phase II study respectively showed that in melanoma and glioma, compared with adjuvant therapy, neoadjuvant immunotherapy could bring more obvious overall survival benefit (23,24). In 2018, Forde et al. first reported the efficacy and feasibility of neoadjuvant immunotherapy for NSCLC (CheckMate159 study), then finding that neoadjuvant immunotherapy could achieve 43% MPR (15).
In the present study, we found that compared to neoadjuvant chemotherapy, ICIs neoadjuvant therapy alone showed a lower incidence of TRAE (25-27), which was similar to the results of previous adjuvant therapy for advanced NSCLC (28,29). Furthermore, the incidence of immune-related adverse reactions above grade 3 is low, mainly immune pneumonia and adrenal insufficiency. Few fatal irAE were reported in our study, mainly caused by immune pneumonia (16). The results of the phase II single-arm study (LCMC3 study) showed that using atezolizumab for neoadjuvant treatment of stage IB–IIIA or resectable stage IIIB NSCLC resulted in only 5.9% (6/101) of patients ≥ grade 3 TRAEs (30). Also, more TRAE occurred in patients undergoing ICI and chemotherapy. Apart from the possibility of chemotherapy-related AEs, we also observed more grade 3 or higher irAEs in the neoadjuvant immunochemotherapy group, suggesting that, in addition to the adverse reactions caused by chemotherapy, the combination therapy may increase the adverse events induced by ICIs.
In terms of surgical resection rate, all but one study showed a surgical resection rate higher than 65% (17), which was not significantly different from the results of neoadjuvant chemotherapy (27); 3 studies showed a 100% surgical resection rate (13,14,18). Concerning surgical delay, only 2/358 patients experienced a surgical delay, mainly due to one case of grade 2 alanine transaminase and aspartate transaminase elevation and a case of grade 1 hyperthyroidism (16). In contrast, a previous article (31) reported that the surgical delay rate during neoadjuvant chemotherapy was relatively high. Although there is no statistically significant difference, it still suggests the advantages of neoadjuvant immunotherapy. We also observed that compared to the neoadjuvant ICI treatment group, more postoperative complications occurred in the ICI combined immunotherapy group (18.9%), with rare fatal complications. Combined with the published literature reporting that the incidence of severe postoperative complications caused by neoadjuvant chemotherapy is about 1–7% (32), we believe that compared to chemotherapy, ICI therapy is safer and has greater advantages in terms of postoperative complications.
Our study reported that in the neoadjuvant immunochemotherapy group, the MPR (68.1%) of the patients were significantly higher than those in the neoadjuvant immunotherapy group alone. Similarly, concerning the RECIST1.1 assessment, the patient’s radiological response rate also showed the same trend. Whereas excluding a study with a small sample size (18), the MRP and pCR rate of the ICI neoadjuvant therapy group were only 15–35%, and the ORR was only 9–20%. Taken together, this suggests that chemotherapy combined with immunotherapy has a greater advantage in the neoadjuvant treatment of NSCLC.
This study is a meta-analysis that evaluated the safety and feasibility of ICIs in neoadjuvant therapy of NSCLC. The previous systemic review of Zhao et al. (32). mainly involved conference abstracts. Given the current high treatment efficiency of ICI combined with chemotherapy, we believe that more efforts should be put into neoadjuvant immunochemotherapy. In addition, through the transformation of the data format, we conducted a meta-analysis of the included single-arm studies for more reliable data.
However, there are some limitations. First, the small sample size limits the reliability of the research. The P value (0.14) in pCR was greater than 0.05, which could not be considered significant. Although there are differences in trends between the two subgroups (I2=53.9%), further high-quality studies are needed. As the included studies were mostly open single-arm clinical trials, the lack of double-blind comparative studies may also increase the risk of bias. Finally, the study lacks follow-up data, so it is difficult to judge the benefits for the long-term survival of patients.
Conclusions
Neoadjuvant immunotherapy of resectable NSCLC shows better clinical application prospects due to its lower toxicity and fewer perioperative complications. Meanwhile, ICI combined chemotherapy can achieve more pathological relief and clinical benefits in the neoadjuvant treatment of NSCLC, but correspondingly, it will increase irAE and perioperative complications, thus more clinical studies are needed to determine the optimal neoadjuvant treatment plan.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1664/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1664/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1664/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Boyd JA, Hubbs JL, Kim DW, et al. Timing of local and distant failure in resected lung cancer: implications for reported rates of local failure. J Thorac Oncol 2010;5:211-4. [Crossref] [PubMed]
- Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 2010;28:29-34. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw 2021;19:254-66. [Crossref] [PubMed]
- Gettinger S, Horn L, Jackman D, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J Clin Oncol 2018;36:1675-84. [Crossref] [PubMed]
- Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year Overall Survival for Patients With Advanced Non‒Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol 2019;37:2518-27. [Crossref] [PubMed]
- Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413-22. [Crossref] [PubMed]
- Tfayli A, Al Assaad M, Fakhri G, et al. Neoadjuvant chemotherapy and Avelumab in early stage resectable nonsmall cell lung cancer. Cancer Med 2020;9:8406-11. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Higgins JPT, Thomas J, Chandler J, et al. editors. Cochrane handbook for systematic reviews of interventions. 2nd edition. Hoboken, NJ: Wiley-Blackwell; 2020.
- Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:269-76. [Crossref] [PubMed]
- Cascone T, William WN Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504-14. [Crossref] [PubMed]
- Chen T, Ning J, Campisi A, et al. Neoadjuvant PD-1 Inhibitors and Chemotherapy for Locally Advanced NSCLC: A Retrospective Study. Ann Thorac Surg 2022;113:993-9. [Crossref] [PubMed]
- Chen Y, Yan B, Xu F, et al. Neoadjuvant chemoimmunotherapy in resectable stage IIIA/IIIB non-small cell lung cancer. Transl Lung Cancer Res 2021;10:2193-204. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2020;15:816-26. [Crossref] [PubMed]
- Liu YT, Gao YS, Mao YS, et al. The outcome and safety of neoadjuvant PD-1 blockade plus chemotherapy in stage II~III non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2020;42:480-5. [PubMed]
- Lücke E, Ganzert C, Föllner S, et al. Operability and Pathological Response of Non-Small Cell Lung Cancer (NSCLC) after Neoadjuvant Therapy with Immune Checkpoint Inhibition. Pneumologie 2020;74:766-72. [PubMed]
- Reuss JE, Anagnostou V, Cottrell TR, et al. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J Immunother Cancer 2020;8:e001282. [Crossref] [PubMed]
- Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786-95. [Crossref] [PubMed]
- Rothschild SI, Zippelius A, Eboulet EI, et al. SAKK 16/14: Durvalumab in Addition to Neoadjuvant Chemotherapy in Patients With Stage IIIA(N2) Non-Small-Cell Lung Cancer-A Multicenter Single-Arm Phase II Trial. J Clin Oncol 2021;39:2872-80. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med 2018;24:1655-61. [Crossref] [PubMed]
- Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 2019;25:477-86. [Crossref] [PubMed]
- Rosell R, Gómez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 1994;330:153-8. [Crossref] [PubMed]
- Betticher DC, Hsu Schmitz SF, Tötsch M, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol 2003;21:1752-9. [Crossref] [PubMed]
- Jia XH, Xu H, Geng LY, et al. Efficacy and safety of neoadjuvant immunotherapy in resectable nonsmall cell lung cancer: A meta-analysis. Lung Cancer 2020;147:143-53. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Kwiatkowski DJ, Rusch VW, Chaft JE, et al. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Interim analysis and biomarker data from a multicenter study (LCMC3). J Clin Oncol 2019;37:abstr 8503.
- Sawabata N, Keller SM, Matsumura A, et al. The impact of residual multi-level N2 disease after induction therapy for non-small cell lung cancer. Lung Cancer 2003;42:69-77. [Crossref] [PubMed]
- Zhao Z, Gao Y, Xue Q, et al. Safety and Efficacy of Neoadjuvant Immune Checkpoint Inhibitor Therapy in Patients with Resectable Non-small-Cell Lung Cancer: A Systematic Review. Target Oncol 2021;16:425-34. [Crossref] [PubMed]