Efficacy of flaxseed oil compared with fish oil supplementation in the treatment of coronary heart disease: a retrospective study
Introduction
Type 2 diabetes mellitus (T2DM) is a major risk factor for coronary heart disease (CHD) (1). It has been reported that T2DM patients have a 2–4 times higher risk of CHD than the general population (2). In recent years, CHD has become the leading cause of death for diabetic patients (3). Insulin resistance not only aggravates hyperglycemia in these patients but also increases the risk of cardiovascular disease (4). Additionally, in the development of atherosclerotic diseases, insulin resistance and other metabolic abnormalities (such as dyslipidemia) will lead to more severe inflammation and excessive production of free radicals (5-8). Reactive oxygen species and free radicals released during atherosclerosis are reported to induce the formation of a highly atherogenic molecule, which accelerates lipid deposition in plaques (9).
Numerous studies have demonstrated the beneficial effects of omega‐3 fatty acids in animal models and human diseases (10). These effects include enhancing eicosanoid metabolism, improving platelet lipid composition, reducing insulin resistance, improving blood lipid levels, and optimizing blood viscosity, blood pressure, blood coagulation, and cytokine and growth factor levels (10-12). Although fish oil may provide health benefits, patients’ compliance with fish oil is often poor due to the unpleasant taste, which can cause nausea and vomiting. Due to the concerns about the palatability of fish oil, it is necessary to find alternative sources of omega-3 fatty acids. Flaxseed oil contains 50% polyunsaturated fatty acids (PUFAs), of which α-Linolenic acid is one of the most abundant vegetarian sources (13). There is growing evidence that flaxseed is a beneficial dietary supplement to maintain healthy cholesterol levels (14,15). When linseed oil was added to the diet of healthy volunteers, their levels of total cholesterol and low-density lipoprotein cholesterol were moderately reduced (16,17). The following is a summary of current data available for comparing the effects of the two different sources of omega‐3 fatty acids: flaxseed oil and fish oil on cardiovascular parameters in T2DM patients with CHD. In a recent clinical trial, overweight diabetic schoolchildren who received 1 month of omega-3 supplementation from a fish oil source (2.4 g per day, containing 480 mg docosahexaenoic acid and 720 mg eicosapentaenoic acid) showed significantly improved blood lipid status and a reduction in fasting glucose levels and blood pressure (18). Moreover, 3 g fish oil supplements every day for 18 months effectively reduced the blood glucose and triglyceride levels of patients with impaired glucose tolerance and improved their levels of high-density lipoprotein cholesterol (19). It was also reported that a daily supplement of 1 g of omega-3 fatty acid extracted from flaxseed oil, which contains 400 mg α‐Linolenic acid, significantly increased insulin levels and insulin sensitivity and reduced triglyceride and very-low-density lipoprotein cholesterol levels, although it did not affect biomarkers of inflammation and oxidative stress (20). There was also a randomized controlled study comparing flaxseed oil and fish oil supplementation, however, the sample size was relatively small and we would include more patients in the present study (21).
Taking into account the anti-inflammatory and antioxidant effects of omega-3 fatty acids and the diversity of available omega-3 sources, we hypothesized that supplementation of omega-3 fatty acids from fish oil and flaxseed oil may be beneficial for T2DM patients with CHD. Considering the anti-inflammatory and antioxidant effects of omega-3 fatty acids and the diversity of available omega-3 sources, we hypothesized that supplementation with omega-3 fatty acids from fish oil and flaxseed oil might benefit T2DM patients with CHD. Therefore, this study aimed to compare the effects of two different sources of omega-3 fatty acids, fish oil and flaxseed oil supplements, on various metabolic parameters in T2DM patients with CHD.
We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-26/rc) (22).
Methods
Patients
This was a retrospective study based on the prospectively maintained database of Hubei Provincial Hospital of Traditional Chinese Medicine. Ethical approval and individual consent for this retrospective analysis were waived by Hubei Provincial Hospital of Traditional Chinese Medicine. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). We searched the database for all patients aged between 40 and 100 years diagnosed with both CHD and T2DM. The criteria of the American Diabetes Association were used for the diagnosis of T2DM (23), while CHD was diagnosed based on the American Heart Association definition (24). Individuals who had taken omega-3 supplements within the past 3 months or had cardiac surgery or acute myocardial infarction within the past 3 months were excluded from the study.
Interventions
Enrolled patients were divided into two groups: the flaxseed oil group received 1,000 mg flaxseed oil, which contains 400 mg of α-Linolenic acid, as the source of their omega‐3 fatty acids; the fish oil group received 1,000 mg of fish oil, which contains 250 mg of eicosapentaenoic acid and 150 mg of docosahexaenoic acid. The dose of fish oil was based on previously published studies on healthy males (25) and women with polycystic ovary syndrome (26). The dose of flaxseed oil was based on a previous study of diabetic patients with foot ulcers (20). To improve compliance, all individuals received text messages on their mobile phones reminding them to take the supplements every day. A 3‐day food record was obtained every three weeks from the beginning to the end of the intervention. Conventional titration was used to determine the dose of flaxseed and fish oil.
Outcomes
Dependent variables in the present study included lipid profiles, glucose homeostasis parameters, and biomarkers of inflammation and oxidative stress. The primary outcome was the homeostatic model assessment for insulin resistance (HOMA-IR), and other metabolic indicators were defined as secondary outcomes. Overnight fasting blood (10 mL) was collected at baseline and the end of follow-up. Blood was collected in two separate tubes: one tube containing EDTA was used to detect plasma total nitrite levels and biomarkers of oxidative stress, while the other tube without EDTA was used to separate the serum to quantify lipid profiles, serum insulin, and high‐sensitivity C‐reactive protein (hs‐CRP) concentrations. The samples were kept at −80 °C until the final analysis. The level of insulin was measured by an ELISA Kit (DiaMetra, Milano, Italy). The coefficients of variation (CV) inter‐ and intra‐assay were 3.1% and 4.9%, respectively. The standard formulas (27) were used to test the HOMA-IR and quantitative insulin sensitivity index (QUICKI). Lipid profiles (triglycerides, very low-density lipoprotein, low-density lipoprotein, high-density lipoprotein, and total cholesterol) and fasting plasma glucose (FPG) were measured using enzymatic kits (Pars Azmun, Tehran, Iran), with inter‐ and intra‐assay CVs less than 5%. A commercial ELISA kit (LDN, Nordhorn, Germany) was used to measure the serum hs‐CRP concentrations, and the inter‐ and intra‐assay CVs were 4.4% and 6.6%, respectively. Survival data were also compared between the two groups of patients. Patients who lost to follow-up were excluded from the analyses.
Statistical analysis
The Kolmogorov-Smirnov test was performed to assess whether variables were normally distributed. A log transformation was used for any non-normally distributed variables. The differences in dietary intake and general characteristics between the two groups were detected using a one‐way analysis of variance. Categorical variables were compared using the Pearson chi-square test. Potential bias was avoided by controlling all analyses for baseline values, age, and baseline body mass index (BMI). P values less than 0.05 were considered statistically significant. All the above analyses were performed using STATA (version 16, Stata SE).
Results
As shown in Figure 1, A total of 177 T2DM patients [mean (SD) age, 53.2 (9.6) years; 94 (53.1%) male and 83 (46.9%) female] with CHD were screened out from the database of Hubei Provincial Hospital of Traditional Chinese Medicine. Among them, 92 patients received flaxseed oil, and 85 patients received fish oil. After eligibility evaluation, 54 patients were excluded for various reasons. Finally, we included 120 patients in the present retrospective study: 60 patients in the flaxseed oil group and 60 patients in the fish oil group. As shown in Table 1, although a significant difference was observed in total energy intakes in the male subgroups (2,512±92 vs. 2,498±151, P=0.02) and protein intakes in the female subgroups (15.7±3.6 vs. 13.1±2.1, P=0.01), the baseline dietary intake of all subjects was generally balanced between the two groups.
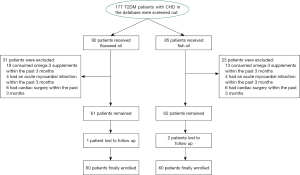
Table 1
| Variables | Flaxseed oil (n=60) | Fish oil (n=60) | P values* | |||
|---|---|---|---|---|---|---|
| Means | SD | Means | SD | |||
| Energy (kcal/d) | ||||||
| Combined | 2,369 | 252 | 2,310 | 211 | 0.44 | |
| Female | 2,201 | 258 | 2,159 | 119 | 0.81 | |
| Male | 2,512 | 92 | 2,498 | 151 | 0.02 | |
| Carbohydrates (%) | ||||||
| Combined | 53.9 | 6.6 | 56.2 | 7.4 | 0.61 | |
| Female | 52.8 | 7.6 | 57.3 | 8.1 | 0.09 | |
| Male | 55.1 | 6.2 | 55.1 | 6.2 | 0.89 | |
| Protein (%) | ||||||
| Combined | 15.2 | 3.1 | 13.6 | 1.7 | 0.21 | |
| Female | 15.7 | 3.6 | 13.1 | 2.1 | 0.01 | |
| Male | 14.1 | 2.2 | 14.2 | 2.5 | 0.77 | |
| Omega‐3 (%) | ||||||
| Combined | 0.3 | 0.1 | 0.3 | 0.1 | 0.24 | |
| Female | 0.4 | 0.1 | 0.4 | 0.1 | 0.51 | |
| Male | 0.5 | 0.2 | 0.4 | 0.1 | 0.69 | |
| Fat (%) | ||||||
| Combined | 31.1 | 5.2 | 30.2 | 6.1 | 0.56 | |
| Female | 30.8 | 4.9 | 29.9 | 7.1 | 0.32 | |
| Male | 31.4 | 5.3 | 31.1 | 4.8 | 0.78 | |
| PUFA (%) | ||||||
| Combined | 10.3 | 2.6 | 10.9 | 2.2 | 0.88 | |
| Female | 9.9 | 2.1 | 9.6 | 1.9 | 0.36 | |
| Male | 10.3 | 2.2 | 11.2 | 2.4 | 0.22 | |
| MUFA (%) | ||||||
| Combined | 8.6 | 2.4 | 8.4 | 2.3 | 0.43 | |
| Female | 7.7 | 2.3 | 7.7 | 2.3 | 0.87 | |
| Male | 8.8 | 2.2 | 8.7 | 2.1 | 0.08 | |
| SFA (%) | ||||||
| Combined | 9.8 | 1.9 | 8.8 | 2.3 | 0.28 | |
| Female | 9.3 | 2.2 | 8.4 | 3.1 | 0.22 | |
| Male | 10.1 | 2.4 | 9.9 | 1.5 | 0.55 | |
| AOAC TDF (g/d) | 17.6 | 2.8 | 18.4 | 2.9 | 0.28 | |
| Cholesterol (mg/d) | 231.5 | 131.2 | 196.7 | 127.1 | 0.59 | |
*, P values were derived from the ANOVA test. PUFA, polyunsaturated fatty acid; MUFA, monounsaturated fatty acid; SFA, saturated fatty acid; AOAC, Association of Official Analytical Chemists; TDF, total dietary fiber.
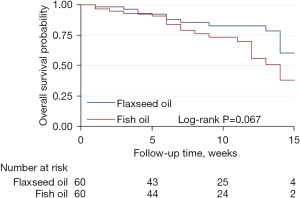
Figure 2 shows the changes in cardiometabolic risk biomarkers at 0, 3, 6, 9, 12, and 15 weeks of follow-up. After a median follow-up time of 10.00 months (95% CI: 8.42–11.58 months), There were no significant differences in HOMA-IR, FPG, body weight, or BMI between the flaxseed oil and fish oil groups. However, flaxseed oil was observed to have a significantly better effect than fish oil for reducing serum insulin levels and hs‐CRP levels (P=0.03 and P=0.02, respectively). After a median follow-up of 10.0 weeks (95% CI: 8.4–11.6 weeks), patients who received flaxseed oil tended to have a better overall survival than those who received fish oil, although the difference was not statistically significant (P=0.067), as shown in Figure 3.

Table 2 shows the patterns of adverse events and causes of death between the two groups. Dizziness, asthma, and vomiting were common adverse events among the study participants. In general, the incidence of adverse events was significantly lower in patients who received flaxseed oil than in those who received fish oil (P=0.039). Causes of death observed in the present study included respiratory failure, myocardial infarction, diabetic ketoacidosis, and cerebral hemorrhage. However, the difference in overall mortality was not statistically significant between the two groups (P=0.144).
Table 2
| Variables | Flaxseed oil (n=60) | Fish oil (n=60) | P values* | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Adverse events | 0.039 | |||||
| Dizziness | 3 | 5.0 | 3 | 5.0 | ||
| Asthma | 2 | 3.3 | 7 | 11.7 | ||
| Vomiting | 4 | 6.7 | 6 | 10.0 | ||
| Coma | 0 | 0 | 1 | 1.7 | ||
| Others | 2 | 3.3 | 4 | 6.7 | ||
| Causes of death | 0.144 | |||||
| Respiratory failure | 2 | 3.3 | 3 | 5.0 | ||
| Myocardial infarction | 6 | 10.0 | 9 | 15.0 | ||
| Diabetic ketoacidosis | 1 | 1.7 | 2 | 3.3 | ||
| Cerebral hemorrhage | 2 | 3.3 | 3 | 5.0 | ||
| Unknown | 1 | 1.7 | 2 | 3.3 | ||
*, P values were tested by the chi-square test.
Discussion
Our results revealed that flaxseed oil was more effective in reducing insulin and hs‐CRP levels than fish oil, although both flaxseed oil and fish oil had similar effects on other cardiovascular risk biomarkers. However, flaxseed oil tended to improve the overall survival rate when compared with fish oil.
It was reported in a previous study that in diabetic patients with nephropathy, supplementation with omega-3 fatty acids from flaxseed oil at 1,000 mg/day dose for 12 weeks significantly reduced serum insulin, triglyceride, and very-low‐density lipoprotein cholesterol levels and simultaneously increased insulin sensitivity (20). In addition, obese subjects who received omega-3 fatty acids (1.8 g/day) from fish oil for 12 weeks showed a reduction in their levels of serum insulin and triglycerides but other blood glucose control indicators and blood lipid levels remained unchanged (28). However, there are inconsistent results in the literature; for example, in one trial, a 60 mg/kg/day dose of flaxseed oil supplementation for 3 months did not significantly affect HOMA-IR in T2DM (29). Another study found that 8 weeks of continuous omega-3 fatty acid intake (6 g/day fish oil) for T2DM patients reduced their triglyceride levels, but their glucose metabolism was not influenced (30). The observed inconsistent results may be due to the oil dosage, the formulations used, or even the study durations.
As for CRP levels, and in line with our findings, omega-3 fatty acid supplementation (720 mg of eicosapentaenoic acid and 480 mg of docosahexaenoic acid) was reported to significantly reduce hs‐CRP levels for patients with cardiovascular disease (31). A further study reported that supplementation with omega-3 fatty acids (1,080 mg of eicosapentaenoic acid and 200 mg of docosahexaenoic acid) for women with systemic lupus erythematosus effectively reduced CRP levels (32). Soleimani et al. also documented that a 2 g/day dose of omega-3 fatty acid supplementation from flaxseed oil for 12 weeks significantly reduced serum hs-CRP levels (33).
The small sample size and short follow-up are the main limitations of our study. Therefore, further well-designed studies with larger sample sizes and a longer follow-up are needed to verify the results observed in this study.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-26/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-26/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-26/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This was a retrospective study based on the prospectively maintained database of Hubei Provincial Hospital of Traditional Chinese Medicine. Ethical approval and individual consent for this retrospective analysis were waived by Hubei Provincial Hospital of Traditional Chinese Medicine.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bundhun PK, Bhurtu A, Yuan J. Impact of type 2 diabetes mellitus on the long-term mortality in patients who were treated by coronary artery bypass surgery: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7022. [Crossref] [PubMed]
- Booth GL, Kapral MK, Fung K, et al. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 2006;368:29-36. [Crossref] [PubMed]
- Micha R, Peñalvo JL, Cudhea F, et al. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017;317:912-24. [Crossref] [PubMed]
- Abdul-Ghani MA, Jayyousi A, DeFronzo RA, et al. Insulin Resistance the Link between T2DM and CVD: Basic Mechanisms and Clinical Implications. Curr Vasc Pharmacol 2019;17:153-63. [Crossref] [PubMed]
- Paneni F, Beckman JA, Creager MA, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J 2013;34:2436-43. [Crossref] [PubMed]
- Paneni F, Costantino S, Cosentino F. Molecular mechanisms of vascular dysfunction and cardiovascular biomarkers in type 2 diabetes. Cardiovasc Diagn Ther 2014;4:324-32. [PubMed]
- von Bibra H, St John Sutton M, Schuster T, et al. Oxidative stress after a carbohydrate meal contributes to the deterioration of diastolic cardiac function in nonhypertensive insulin-treated patients with moderately well controlled type 2 diabetes. Horm Metab Res 2013;45:449-55. [Crossref] [PubMed]
- Zhang X, Yan SM, Zheng HL, et al. A mechanism underlying hypertensive occurrence in the metabolic syndrome: cooperative effect of oxidative stress and calcium accumulation in vascular smooth muscle cells. Horm Metab Res 2014;46:126-32. [PubMed]
- Ross R. Atherosclerosis is an inflammatory disease. Am Heart J 1999;138:S419-20. [Crossref] [PubMed]
- Deckelbaum RJ, Leaf A, Mozaffarian D, et al. Conclusions and recommendations from the symposium, Beyond Cholesterol: Prevention and Treatment of Coronary Heart Disease with n-3 Fatty Acids. Am J Clin Nutr 2008;87:2010S-2S. [Crossref] [PubMed]
- Brox JH, Killie JE, Osterud B, et al. Effects of cod liver oil on platelets and coagulation in familial hypercholesterolemia (type IIa). Acta Med Scand 1983;213:137-44. [Crossref] [PubMed]
- Mori TA, Vandongen R, Beilin LJ, et al. Effects of varying dietary fat, fish, and fish oils on blood lipids in a randomized controlled trial in men at risk of heart disease. Am J Clin Nutr 1994;59:1060-8. [Crossref] [PubMed]
- Raper NR, Cronin FJ, Exler J. Omega-3 fatty acid content of the US food supply. J Am Coll Nutr 1992;11:304-8. [Crossref] [PubMed]
- Prasad K. Regression of hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseed. Atherosclerosis 2008;197:34-42. [Crossref] [PubMed]
- Prasad K, Jadhav A. Prevention and treatment of atherosclerosis with flaxseed-derived compound secoisolariciresinol diglucoside. Curr Pharm Des 2016;22:214-20. [Crossref] [PubMed]
- Cunnane SC, Ganguli S, Menard C, et al. High alpha-linolenic acid flaxseed (Linum usitatissimum): some nutritional properties in humans. Br J Nutr 1993;69:443-53. [Crossref] [PubMed]
- Cunnane SC, Hamadeh MJ, Liede AC, et al. Nutritional attributes of traditional flaxseed in healthy young adults. Am J Clin Nutr 1995;61:62-8. [Crossref] [PubMed]
- García-López S, Villanueva Arriaga RE, Nájera Medina O, et al. One month of omega-3 fatty acid supplementation improves lipid profiles, glucose levels and blood pressure in overweight schoolchildren with metabolic syndrome. J Pediatr Endocrinol Metab 2016;29:1143-50. [Crossref] [PubMed]
- Derosa G, Cicero AF, D'Angelo A, et al. Effects of n-3 pufas on fasting plasma glucose and insulin resistance in patients with impaired fasting glucose or impaired glucose tolerance. Biofactors 2016;42:316-22. [PubMed]
- Soleimani A, Taghizadeh M, Bahmani F, et al. Metabolic response to omega-3 fatty acid supplementation in patients with diabetic nephropathy: A randomized, double-blind, placebo-controlled trial. Clin Nutr 2017;36:79-84. [Crossref] [PubMed]
- Raygan F, Taghizadeh M, Mirhosseini N, et al. A comparison between the effects of flaxseed oil and fish oil supplementation on cardiovascular health in type 2 diabetic patients with coronary heart disease: A randomized, double-blinded, placebo-controlled trial. Phytother Res 2019;33:1943-51. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. [Crossref] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36:S67-74. [Crossref] [PubMed]
- Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003;108:2543-9. [Crossref] [PubMed]
- Tsuchiya Y, Yanagimoto K, Nakazato K, et al. Eicosapentaenoic and docosahexaenoic acids-rich fish oil supplementation attenuates strength loss and limited joint range of motion after eccentric contractions: a randomized, double-blind, placebo-controlled, parallel-group trial. Eur J Appl Physiol 2016;116:1179-88. [Crossref] [PubMed]
- Oner G, Muderris II. Efficacy of omega-3 in the treatment of polycystic ovary syndrome. J Obstet Gynaecol 2013;33:289-91. [Crossref] [PubMed]
- Pisprasert V, Ingram KH, Lopez-Davila MF, et al. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care 2013;36:845-53. [Crossref] [PubMed]
- Polus A, Zapala B, Razny U, et al. Omega-3 fatty acid supplementation influences the whole blood transcriptome in women with obesity, associated with pro-resolving lipid mediator production. Biochim Biophys Acta 2016;1861:1746-55. [Crossref] [PubMed]
- Barre DE, Mizier-Barre KA, Griscti O, et al. Flaxseed oil supplementation manipulates correlations between serum individual mol % free fatty acid levels and insulin resistance in type 2 diabetics. Insulin resistance and percent remaining pancreatic β-cell function are unaffected. Endocr Regul 2016;50:183-93. [Crossref] [PubMed]
- Luo J, Rizkalla SW, Vidal H, et al. Moderate intake of n-3 fatty acids for 2 months has no detrimental effect on glucose metabolism and could ameliorate the lipid profile in type 2 diabetic men. Results of a controlled study. Diabetes Care 1998;21:717-24. [Crossref] [PubMed]
- Agh F, Mohammadzadeh Honarvar N, Djalali M, et al. Omega-3 Fatty Acid Could Increase One of Myokines in Male Patients with Coronary Artery Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Arch Iran Med 2017;20:28-33. [PubMed]
- Curado Borges M, de Miranda Moura Dos Santos F, Weiss Telles R, et al. Omega-3 fatty acids, inflammatory status and biochemical markers of patients with systemic lupus erythematosus: a pilot study. Rev Bras Reumatol Engl Ed 2017;57:526-34. [Crossref] [PubMed]
- Soleimani Z, Hashemdokht F, Bahmani F, et al. Clinical and metabolic response to flaxseed oil omega-3 fatty acids supplementation in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. J Diabetes Complications 2017;31:1394-400. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)