Endobronchial ultrasound elastography: a new method in endobronchial ultrasound-guided transbronchial needle aspiration
Background
Non-small cell lung cancer (NSCLC) comprises 80% to 85% of lung cancer, with most patients presenting with advanced-stage disease with an overall of only 5% (1). The NCCN Guidelines for NSCLC recommends molecular testing for genetic mutations, such as ALK and EGFR mutations, so as to consider treatment with appropriately targeted agents, for example crizotinib in ALK-positive disease or erlotinib for sensitizing EGFR mutation-positive disease (2). Obtaining adequate specimens is the key to provide a specific histologic diagnosis and allow appropriate molecular testing of patients with thoracic malignancy (3). Conventional TBNA and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) are generally considered to be first-line tools for obtaining specimens of mediastinal and hilar lymph nodes (4-6). EGFR mutation and ALK gene rearrangement analysis in EBUS-guided needle aspirates using pyrosequencing and fish analysis are feasible and show a high diagnostic success rate (7-9).
The needle techniques of endobronchial ultrasound-needle aspiration, endoscopic ultrasound-needle aspiration, and combined endobronchial ultrasound/endoscopic ultrasound-needle aspiration have sensitivities of approximately 89%, 89%, and 91%, respectively (10).
Thus, EBUS-TBNA technology in lung cancer staging and diagnosis are very important and physicians will need to be skilled in the technology (11).
Methods
EBUS can be utilized in peribronchial and mediastinal lesions with the help of ultrasonography features and the operator must, therefore, master EBUS-ultrasonography and the use of the TBNA needle (12). In the 10 years since the first introduction of EBUS-TBNA into clinical practice, the Olympus Company has developed the second generation EBUS (BF-UC260FW) and a new ultrasound image processor (EU-M2) (13). Also, FUJI company has developed a curvilinear array endobronchial ultrasound bronchoscope (EB-530 US) (14). A new thin convex probe endobronchial ultrasound bronchoscope (TCP-EBUS; Olympus Co.) is also in development (15). The emergence of new equipment is accompanied by new diagnostic methods, which can lead to improvements in the diagnostic yield of lung cancer. In this manuscript, we will review the current technology of EBUS-TBNA, focusing on the development of new innovations in convex probe (CP)-EBUS and instruments.
Results
The new generation CP-EBUS
Endobronchial ultrasound has emerged as a cutting-edge technology that allows the bronchoscopist to see beyond the airway. The CP-EBUS was designed to perform real-time EBUS-guided TBNA. C-TBNA and particularly EBUS-TBNA have become first-line tools for the staging and diagnosis of patients with (suspected) lung cancer.
At present, there are three companies that have developed CP-EBUS with their respective bronchoscope and ultrasonic functions are shown in Table 1.
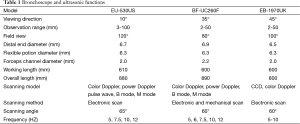
Full table
Innovation of forceps channel diameter
Due to the increase of the second generation of CP-EBUS bronchial working channel size, in addition to the two types of dedicated EBUS-TBNA needles (NA-201SX-4021 and NA-201SX-4022), the Wang-319 histologic needle, and the Cook needle (Echo-HD-EBUS, Echo-HD-19) are able to pass through the 2.2 mm forceps channel.
Indications for EBUS-TBNA are for the assessment of mediastinal and hilar lymph to aid in the staging and diagnosis of thoracic malignancy. As for the influence of needle size on the diagnostic yield of EBUS-TBNA in lung cancer, we refer to the related literature. Masahide Oki carried out a clinical randomized study comparing 21 g and 22 g histological specimens with the sampling yield of adequate histologic specimens obtained by the 21-gauge and 22-gauge needles being 72% and 78% (P=0.40), respectively (16). Nakajima et al. reported their clinical trial from 33 patients evaluated by EBUS-TBNA. Within the 23 malignant sites, tumour cells were equally detected by both 21G and 22G needles (diagnostic accuracy rate was both 100% for each needle in this study) (17). In addition, the American College of Chest Physicians Quality Improvement Registry retrospectively evaluated the results of 1,299 patients. Sample adequacy was obtained in 94.9% of the 22G needle group and in 94.6% of the 21G needle group (P=0.81). A diagnosis was made in 51.4% of the 22G and 51.3% of the 21G groups (P=0.98) (18). To sum up, there were no differences in the diagnostic yield between the 21G and 22G needles during EBUS-TBNA. Therefore, guidelines for the acquisition specimens indicated needle type or needle size did not affect the diagnostic yield (3).
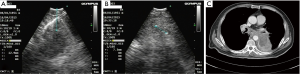
Permanent radioactive iodine-125 (125I) seed implantation has been an option for treatment of selected patients with prostate cancer with 125I being a feasible radiation source that can be permanently implanted into the tumor sites (19). Luo et al. reported 43 patients with mediastinal malignant tumors treated with CT-guided radiation seed implantation in mediastinal malignant tumors as a relatively safe technique with high success rate (20). Qiming Huang demonstrated that percutaneous interstitial brachytherapy using CT-guided 125I radioactive seeds is both feasible and effective for patients with malignant thoracic tumors (21). The 125I radioactive seed has a diameter of 0.8 mm and length of 4.5 mm and needs to pass through an 18 g needle. Because of the special anatomical location position of mediastinal lesions, we believe that puncturing the mediastinal lesions by the EBUS-TBNA technique is safer than a percutaneous puncture technique. Therefore, we used EBUS-TBNA technology to implant a 125I radioactive seed to treat mediastinal lymph nodes, and obtained preliminary experience in a 64-year-old man with advanced adenocarcinoma who was treated with EBUS-guided TBNA 125I implantation in a subcarinal lymph node after third- line treatment failure (Figure 1).
Innovation of viewing direction
The directional view of the current EBUS scope is 35-45 degrees. The authors prefer to examine the tracheobronchial tree using the conventional scope, followed by CP-EBUS for EBUS-TBNA. The forward oblique view with the CP-EBUS makes the manipulation of the scope difficult not only for a novice but also for an experienced bronchoscopist.
Yixiang reported using the Fuji EU-530US EBUS scope with the directional view of the Fuji EBUS scope being only 10 degrees so that the vocal cords were easily passed and every lobe able to be entered and examined up to the segmental bronchial openings (14).
Innovation of the flexible portion diameter of the ultrasound bronchoscope
Since the CP-EBUS scope has a larger external diameter than a regular bronchovideoscope and the outer diameter of the tip of the CP-EBUS is 6.9 mm, all of the mediastinal lymph nodes except for station 5 (subaortic or aortopulmonary window), station 6 (para-aortic), station 8 (paraesophageal) and station 9 (pulmonary ligament) are accessible by CP-EBUS. Also, the hilar lymph nodes levels 10 and 11 are approachable, however; part of level 12 is not accessible (22). Wada et al. assessed the thin CP-EBUS (TCP-EBUS) and aspiration needle for sampling of N1 lymph nodes in a porcine model. The TCP-EBUS (BF-Y0046, Olympus Medical Systems Corp.) with a thinner tip (5.9 mm) and larger bending angle (170 degrees upward) was able to visualize one to three distal bifurcations farther compared with the current CP-EBUS. Adequate lymph node sampling from lobar and segmental lymph nodes was possible using the aspiration needle. Thus, they conclude that the TCP-EBUS has improved accessibility to peripheral bronchi with excellent operability and capable of sampling lobar and segmental lymph nodes using the dedicated aspiration needle (15). We look forward to the further clinical trials.
New ultrasound image processor
The second-generation CP-EBUS is compatible with connecting to several kinds of high-quality ultrasound scanners, The EU-ME1 is equipped with the power Doppler mode as well as the color Doppler mode, and the frequency of the ultrasound from 5 to 12 MHz, which makes ultrasound images more clear and allows further study mediastinal lymph nodes by ultrasonographic analysis.
Ultrasonography is a useful imaging tool in the assessment of neck lymph nodes, Gray-scale, power, and color Doppler ultrasonography offers an inexpensive yet effective method in identifying abnormal cervical lymph nodes. Greyscale ultrasonography assesses the size, distribution, and internal architecture of lymph nodes (23,24). Doppler ultrasonography evaluates the intranodal vascular pattern and resistance of lymph nodes (25-28). Contrast-enhanced ultrasonography provides information on lymph node parenchymal perfusion (29). Elastography allows qualitative and quantitative assessment of lymph node stiffness (30).
Fujiwara et al. reported they assessed the utility of themorphologic features of lymph nodes obtained by endobronchial ultrasound. They defined six different morphologic characteristics of mediastinal and hilar lymph nodes during EBUS-TBNA in patients with lung cancer. The lymph nodes were characterized based on EBUS images as follows: (I) short-axis size less or more than 1 cm; (II) shape (oval or round); (III) margin (indistinct or distinct); (IV) echogenicity (homogeneous or heterogeneous); (V) presence or absence of central hilar structure (CHS); and (VI) presence or absence of coagulation necrosis sign. The actual results of comparison between EBUS image classification and final pathology found that lymph nodes with small sizes, round shapes, indistinct margins, homogeneous echogenicities, and the presence of CHS tended to be benign. On the other hand, when lymph nodes had the presence of CHS, they tended to be malignant, and came to the conclusion that sonographic features of lymph nodes based on the new EBUS imaging classification may be helpful in the prediction of metastatic lymph nodes during EBUS-TBNA (31).
Nakajima et al. retrospectively evaluated one hundred and seventy-three mediastinal lymph nodes and concluded vascular image patterns of lymph nodes using power/color Doppler mode is helpful in the prediction of metastatic involvement (32).
Color and power Doppler can allow better detection of vascular involvement before lymph node sampling, then guide the operator avoid suction the blood vessels during TBNA operation, thus avoid the sample too much blood.
Elastography technique
Ultrasound elasticity imaging is an emerging ultrasonic diagnosis technology in recent decades (33). Real-time tissue elastography (RTE) performed during endobronchial ultrasound is a relatively new method for characterizing tissue compliance, RTE with different color shows the difference of tissue after different compression deformation (34,35).
Due to the difference of imaging principle and method, ultrasound elasticity imaging compared with the traditional ultrasonic imaging technology is a new kind of ultrasonic imaging diagnostic technique, which has opened up a new field of ultrasonic diagnosis. Described as E-mode, there is different color elastic coding for different nodal characteristics. The elastic code reflects the nodal hardness with the colors associated with hard, intermediate, and soft tissues being blue, green, and red, Ultrasound devices equipped with the sonoelastography option enable more accurate imaging and evaluation of the nature of lesions situated at small depth, e.g., breast, thyroid, testicles, prostate, and some groups of lymph nodes (30,35).
Now, there are some articles about endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Siokozov reported elastography was carried out using a Pentax EB-1970UK echobronchoscope with a Hitachi Noblus ultrasound scanner and believe endobronchial ultrasound elastography is technically feasible (36). Terunaga Inage reported they used endobronchial ultrasonography elastography to assess whether a tumor has invaded the surrounding structures and concluded that elastography incorporated into an ultrasound system should be more useful for assessing whether a tumor has invaded the surrounding structures than B-mode imaging (37). Andreo García reported their experience about two cases with this technique using an ultrasound bronchoscope (EUM-2) to examine mediastinal lymph nodes. They believe it may be important to categorize the risk of malignancy to facilitate sampling decisions (38). Izumo evaluated seventy-five lymph nodes with a new endoscopic ultrasound processor (EUM-2) to assess elastographic patterns. EBUS elastography sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy rates were demonstrated to be 100%, 92.3%, 94.6%, 100%, and 96.7%, respectively, with the high negative predictive value potentially minimizing unnecessary punctures. They came to the conclusion that endobronchial ultrasound elastography of mediastinal and hilar lymph nodes are a noninvasive technique that can be performed reliably and may be helpful in the prediction of nodal metastasis during EBUS-TBNA (13).
Conclusions
Innovative bronchoscopic techniques allow the diagnosis of lung cancer earlier and more accurately and may, therefore, improve patient outcomes. The further development of EBUS-TBNA, and techniques, such as endobronchial ultrasound elastography of mediastinal and hilar lymph nodes improves diagnostic yield and the negative predictive value.
Acknowledgements
The authors would like to humbly thank Dr. Ko-Pen Wang for his advice in this work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80. [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. Non-Small Cell Lung Cancer, Version 6.2015. J Natl Compr Canc Netw 2015;13:515-24. [PubMed]
- van der Heijden EH, Casal RF, Trisolini R, et al. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014;88:500-17. [PubMed]
- Wang KP, Terry PB. Transbronchial needle aspiration in the diagnosis and staging of bronchogenic carcinoma. Am Rev Respir Dis 1983;127:344-7. [PubMed]
- Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8. [PubMed]
- Herth FJ, Eberhardt R, Vilmann P, et al. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax 2006;61:795-8. [PubMed]
- Nakajima T, Yasufuku K, Suzuki M, et al. Assessment of epidermal growth factor receptor mutation by endobronchial ultrasound-guided transbronchial needle aspiration. Chest 2007;132:597-602. [PubMed]
- Stigt JA, 'tHart NA, Knol AJ, et al. Pyrosequencing analysis of EGFR and KRAS mutations in EUS and EBUS-derived cytologic samples of adenocarcinomas of the lung. J Thorac Oncol 2013;8:1012-8. [PubMed]
- Neat MJ, Foot NJ, Hicks A, et al. ALK rearrangements in EBUS-derived transbronchial needle aspiration cytology in lung cancer. Cytopathology 2013;24:356-64. [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S.
- Yasufuku K, Chiyo M, Koh E, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer 2005;50:347-54. [PubMed]
- Herth FJ, Becker HD, Ernst A. Ultrasound-guided transbronchial needle aspiration: an experience in 242 patients. Chest 2003;123:604-7. [PubMed]
- Izumo T, Sasada S, Chavez C, et al. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol 2014;44:956-62. [PubMed]
- Xiang Y, Zhang F, Akulian J, et al. EBUS-TBNA by a new Fuji EBUS scope (with video). J Thorac Dis 2013;5:36-9. [PubMed]
- Wada H, Hirohashi K, Nakajima T, et al. Assessment of the new thin convex probe endobronchial ultrasound bronchoscope and the dedicated aspiration needle: a preliminary study in the porcine lung. J Bronchology Interv Pulmonol 2015;22:20-7. [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Randomized Study of 21-gauge Versus 22-gauge Endobronchial Ultrasound-guided Transbronchial Needle Aspiration Needles for Sampling Histology Specimens. J Bronchology Interv Pulmonol 2011;18:306-10. [PubMed]
- Nakajima T, Yasufuku K, Takahashi R, et al. Comparison of 21-gauge and 22-gauge aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. Respirology 2011;16:90-4. [PubMed]
- Yarmus LB, Akulian J, Lechtzin N, et al. Comparison of 21-gauge and 22-gauge aspiration needle in endobronchial ultrasound-guidedtransbronchial needle aspiration: results of American College of Chest PhysiciansQuality Improvement Registry, Education, and Evaluation Registry. Chest 2013;143:1036-43. [PubMed]
- Ebara S, Katayama N, Tanimoto R, et al. Iodine-125 seed implantation (permanent brachytherapy) for clinically localized prostate cancer. Acta Med Okayama 2008;62:9-13. [PubMed]
- Luo L, Wang H, Ma H, et al. Radioactive seed implantation for the treatment of mediastinal malignant tumors and lymph node metastases in 43 cases. Zhongguo Fei Ai Za Zhi 2011;14:933-7. [PubMed]
- Huang Q, Chen J, Chen Q, et al. Computed tomographic-guided iodine-125 interstitial implants for malignant thoracic tumors. Eur J Radiol 2013;82:2061-6. [PubMed]
- Adams K, Shah PL, Edmonds L, et al. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757-62. [PubMed]
- Ahuja A, Ying M, King W, et al. A practical approach to ultrasound of cervical lymph nodes. J Laryngol Otol 1997;111:245-56. [PubMed]
- Ying M, Ahuja A, Brook F, et al. Sonographic appearance and distribution of normal cervical lymph nodes in a Chinese population. J Ultrasound Med 1996;15:431-6. [PubMed]
- Ahuja A, Ying M, Yuen YH, et al. Power Doppler sonography of cervical lymphadenopathy. Clin Radiol 2001;56:965-9. [PubMed]
- Wu CH, Chang YL, Hsu WC, et al. Usefulness of Doppler spectral analysis and power Doppler sonography in the differentiation of cervical lymphadenopathies. AJR Am J Roentgenol 1998;171:503-9. [PubMed]
- Ophir J, Alam SK, Garra BS, et al. Elastography: Imaging the elastic properties of soft tissue with ultrasound. J Med Ultrasonics 2002;29:155-171.
- Giovagnorio F, Caiazzo R, Avitto A. Evaluation of vascular patterns of cervical lymph nodes with power Doppler sonography. J Clin Ultrasound 1997;25:71-6. [PubMed]
- Jin Y, He YS, Zhang MM, et al. Value of contrast-enhanced ultrasonography in the differential diagnosis of enlarged lymph nodes: a meta-analysis of diagnostic accuracy studies. Asian Pac J Cancer Prev 2015;16:2361-8. [PubMed]
- Choi YJ, Lee JH, Baek JH. Ultrasound elastography for evaluation of cervical lymph nodes. Ultrasonography 2015;34:157-64. [PubMed]
- Fujiwara T, Yasufuku K, Nakajima T, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest 2010;138:641-7. [PubMed]
- Nakajima T, Anayama T, Shingyoji M, et al. Vascular image patterns of lymph nodes for the prediction of metastatic disease during EBUS-TBNA for mediastinal staging of lung cancer. J Thorac Oncol 2012;7:1009-14. [PubMed]
- Konofagou EE. Quo vadis elasticity imaging? Ultrasonics 2004;42:331-6. [PubMed]
- Ophir J, Alam SK, Garra B, et al. Elastography: ultrasonic estimation and imaging of the elastic properties of tissues. Proc Inst Mech Eng H 1999;213:203-33. [PubMed]
- Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology 2006;239:341-50. [PubMed]
- Sivokozov IV, Silina TL, Korolev VN, et al. The first experience in using elastography in combination with endobronchial ultrasonography for mediastinal pathology: Preliminary assessment of feasibility and comparison of characteristics via different approaches. Vestn Rentgenol Radiol 2014.13-9. [PubMed]
- Inage T, Nakajima T, Yoshida S, et al. Endobronchial elastography in the evaluation of esophageal invasion. J Thorac Cardiovasc Surg 2015;149:576-7. [PubMed]
- Andreo García F, Centeno Clemente CÁ, Sanz Santos J, et al. Initial experience with real-time elastography using an ultrasound bronchoscope for the evaluation of mediastinal lymph nodes. Arch Bronconeumol 2015;51:e8-11. [PubMed]


