Thoracoscopy: medical versus surgical—in the management of pleural diseases
History
The credits of popularizing thoracoscopy in human often go to Dr. Hans-Christian Jacobaeus [1879-1937] who was a Swedish internist (1). However, the first thoracoscopy was performed by Sir Francis Cruise [1834-1906] of the Mater Misericordiae Hospital in Dublin in conjunction with Dr. Samuel Gordon in 1865 (2). Cruise published the technique and result of the procedure in the Dublin Quarterly Journal of Medical Science in 1866, only 6 years after obtaining his M.D. degree (3). In 1901, Georg Kelling [1866-1945], a German physician performed a cystoscope-aided intervention of a dog’s abdomen (4). Kelling also claimed to have performed two successful laparoscopic examinations on humans prior to Jacobaeus, but nonetheless failed to timely publish his experiences.
Jacobaeus is regarded as an important figure in regards to modern laparoscopy and thoracoscopy. He first used a cystoscope in 1910 to perform the first thoracoscopy on a patient with tuberculosis (TB) related pleural adhesions. In 1911 he published an article titled Über die möglichkeit die zystoskopie bei Untersuchung seröser höhlungen anzuwenden (the possibilities for performing cystoscopy in examinations of serous cavities) in the journal Münchner Medizinischen Wochenschrift (5). Jacobaeus understood the possibilities and the limitations of the procedure. He popularized the procedure and described the indications and contraindications of thoracoscopy and published on the different uses and technique of the procedure from 1910s to 1930s. He was an advocate of endoscopic training of internists and stressed the need for specialized instruments for improve visualization and overall performance during thoracoscopic examinations (6-8).
The high prevalence of TB in that era resulted in the rapid adoption of thoracoscopy in the management of pleural/thoracic adhesions and remained a mainstay of therapy until the discovery of streptomycin. After the 1950, thoracoscopy became a rarely performed procedure, particularly in the United States (9). Through this time period, thoracoscopy was largely used to assist in diagnosing pleural effusions and for the purpose of talc or silver nitrate pleurodesis (10,11). Rigid bronchoscopes and mediastinoscopes and specialized fiber-optic rigid scopes were used to examine the thoracic cavity.
By the late 1990s, for the first time, a semi-rigid scope with a flexible end was introduced for the examination of the thoracic cavity (12). The revival of thoracoscopy was mainly associated to the advances in technology and the miniaturization of video cameras. These scopes allowed for a complete study of the desired space, while allowing the performer as well as his assistant to visualize the images on video monitors. Once attached to a fiber-optic telescope, the video camera could provide a useful tool, not only for timely examination, but also teaching and training of other physicians observing and assisting with the procedure. Thoracoscopy that was once abandoned as a procedure in the management of tuberculous pleural adhesions has now gained popularity again and is a commonly used procedure by surgeons as well as internists, for its diagnostic and therapeutic applications in the thoracic cavity.
Procedure
Thoracoscopy is used for a wide variety of surgical procedures in the chest and has largely replaced open thoracotomy as the most commonly used operative method to access the thorax (Figure 1). There are two different methods for thoracoscopy: video-assisted thoracoscopic surgery (VATS) and medical thoracoscopy (MT). There are a number of similarities as well as differences between the two procedures as outlined below.
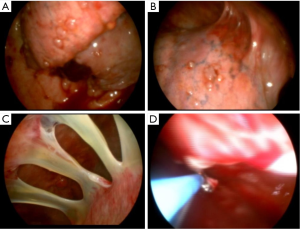
Medical thoracoscopy (MT)
Also known as pleuroscopy, MT can be performed by both surgeons and internists. MT is generally performed under local anesthesia with some premedication. Immediately prior to procedure and after careful review of all available imaging, patients’ pleural space should be examined with ultrasound. While CT scan is an excellent imaging method for the pleura, it has definite limitations. It exposes the patient to ionizing radiation (13) and especially when regular follow-up and imaging is required, US examination is superior compared the chest computed tomography (CT) scan. CT scan images are still images that do not show respiratory variations and are not as easy or accurate as ultrasound in detecting septations in the pleural space (14). Ultrasound is superior in that it is readily available and can be transported to the bedside and is the best modality for guiding pleural procedures such as MT (15). The site of thoracoscope introduction should be selected based on the radiographic images and the area to be examined and biopsied. Attention should be paid to dangerous areas including the internal mammary artery, the subclavian artery coursing in the infraclavicular region and the lateral thoracic artery in the axillary region. The insertion site should be selected after ultrasound examination of the chest to avoid low insertion site and potential injury to diaphragm and intra-abdominal organs. Ultrasound examination of the chest can also help predict procedural success depending on the extent of adhesion and density of the fluid in the pleural space (16,17). It is preferable to examine the chest while the patient is ready and positioned in lateral decubitus state with hemithorax to be examined facing upward.
In general, entry point in marked at the level of fifth to seventh intercostal space, mid to anterior axillary line, for pleural effusions and third to fourth intercostal space for pneumothorax management. The marked area is treated generously with local anesthesia. Sufficient local anesthesia allows for a more successful procedure, decreases the chance of excessive sedative use, potential hypoventilation and discomfort. A small incision in the skin is followed by blunt dissection of the of the chest wall much like a surgical chest tube placement. The trocar is then introduced allowing for the insertion of the thoracoscope.
Thorough examination of the thoracic cavity is dependent on the presence of a sufficiently large space to accommodate and move instruments in the pleural space (18). If the space is occupied by pleural fluid, the fluid should be suctioned out as completely as possible. If there is minimal amount of fluid in the pleural space, a pneumothorax is usually induced by allowing the spontaneously breathing patient to inhale through the open trocar, and occluding the trocar through exhalation. In one study of 29 patients with no pleural effusion, ultrasound guided access to the pleura and biopsy was done safely although eight patients had evidence of multiple adhesions. The pleural space was not fused and diagnosis of malignancy was made in 22 patients. The rest of the patients were found to have nonspecific chronic pleuritis (19). Following detailed examination of the pleural space, biopsies of the parietal pleura are obtained. Two techniques are described when performing MT: single puncture and double puncture (17). In a single puncture method, the thoracoscope is introduced and allows for the introduction of forceps, needle, laser and cautery fibers and allows for suctioning of the pleural space. In the double puncture method, a smaller second insertion site is used to accommodate instruments, while the primary port may be used for visualization. Both rigid and semi-rigid or flex-rigid scopes can be used efficiently for MT.
The advantages of rigid thoracoscopy are the existence of a larger working channel. This working channel can incorporate visualization through a rigid telescope complete with a light source and connected to camera. In addition to efficient visualization however, a major advantage if the use of rigid biopsy forceps which allows the operator to obtain larger biopsy specimens. Larger biopsy specimen may translate to higher diagnostic yield; however, this has not been confirmed in the limited literature comparing rigid vs. flex-rigid scope. Khan et al. compared the diagnostic yield of rigid (27 patients) vs. flex-rigid (39 patients) and found no statistically significant difference (20). Rozman and colleagues showed that the biopsy yield in both rigid and flex-rigid groups in their 84 patients was high (98-100%). They noted no statistically significant difference among two groups, but noted the sample sizes were twice as large in the rigid group (21). One of the disadvantages of rigid thoracoscopy is its potentially increased discomfort to the moderately sedated patient. To thoroughly examine the pleural space, the rigid metal tube needs to be directed in different angles and can apply significant amount of pressure on the ribs, as it is pointed towards different locations in the hemithorax.
The flex-rigid thoracoscope on the other hands is smaller in diameter, and its flexible tip alloes for easier maneuvering between the small rib spaces, while obtaining a thorough examination of the pleural surfaces. Another advantage of the flex-rigid scope is its compatibility with existing processors and light sources routinely available for flexible bronchoscopy. Finally, it is thought to be easier to use by the pulmonologists who are trained to use similar instruments such as the bronchoscope (22,23). On contrary to the rigid thoracoscope, the channel size is the main disadvantage of the flex-rigid scope. The scope can accommodate a small flexible forceps which can obtain smaller specimens. In order to given the advantages and disadvantages of the above scopes, the mini-scope has been used which can provide the operator with a small enough telescope to limit discomfort but accommodate a 3.0 mm forceps biopsy.
Tassi et al., published their experience with mini-thoracoscopy, a technique in which, small 3.8 mm trocar and 3.3 mm telescope are used to direct biopsy of abnormal lesions with a 3.0 mm forceps. They reported a diagnostic yield of 90% in undiagnosed pleural effusions. They found that the most useful attribute of the smaller size scope was its efficiency in diagnosing small effusions that are not accessible to regular size medical thoracoscopes or in patients with small intercostal spaces (24,25).
One of the important aspects of MT is that the procedure is often done in an outpatient setting under moderate sedation. In addition, many institutions perform the procedure in the endoscopy suit as opposed to the operating room. When selected in the right patient population, this results in a significantly reduced cost. In one study by DePew et al., safety and feasibility of MT was assessed in an outpatient setting. A total of 51 patients who had their MT in the outpatient setting were included in to the study. Very few complications were reported. Over 88% of the patients required no further operative interventions beyond those that have been required subsequently regardless of the diagnostic method hence, the avoidance of more invasive initial procedure and hospitalization. It is noteworthy that every selected case in this study, was discussed with a thoracic surgeon and an agreement to proceed was reached prior to outpatient MT (26).
Sedation in MT
As described above, in general, MT is done under moderate sedation and intubation is not required in the setting of spontaneously breathing patient. Cases of significant hypoventilation through MT are however reported in the literature and thought to be secondary to over sedation (26). There are few guidelines for sedation and analgesia by the non-anesthesiologists in the literature (27-30). Despite the number of bronchoscopy and endoscopy procedures performed under moderate sedation, each procedure requires a specific approach. The knowledge of hemodynamic and respiratory changes along with pharmacology of each medication is important prerequisites for optimal application of such medications and varies in different procedures. As noted by Astoul and Maldonado, when it comes to a right choice of sedative for MT, one size does not fit all (31). A combination of a short acting benzodiazepine like midazolam and an opioid like fentanyl are the most common combination used for moderate sedation during MT. Propofol has been used in MT and a randomized noninferiority trial comparing propofol and midazolam was recently published. In this study, 90 patients were randomized to propofol and midazolam. Patients randomized to propofol showed more episodes of hypoxemia (27% vs. 4%, P=0.007) and hypotension (82% vs. 40%, P<0.0001) (32). However, no procedure had to be aborted and none of the patients required an artificial airway, mechanical ventilation or intensive care unit care, and none died. Despite of the lack of sufficient literature on anesthesia and MT, local anesthetic MT is reported to be an overall safe procedure. Rahman et al., reported the pooled results on 22 case series showing a 2% risk of major and 7% risk of minor complications in MT under local anesthesia with moderate sedation (30). The published guidelines also showed a diagnostic yield of 92.6% for malignant pleural diseases.
Video assisted thoracoscopic surgery (VATS)
VATS procedure is generally done under general anesthesia. It is very important to have an anesthesia team experienced in open thoracic procedures as well as single lung ventilation. VATS can be done with double lumen intubation, preferred by most surgeons, or single lumen intubation. However single lumen intubation is generally used when dealing with pleural effusion and parietal pleural biopsy (33). However VATS has also been performed with sedation and local anesthesia (34,35).
The patient is placed on the operating table and the chest is prepped and draped as it would be for a thoracotomy. After induction by general anesthesia, the thoracoscope is inserted and the ipsilateral lung is collapsed for optimal visualization of the intrathoracic structures. After detailed examination of the thoracic cavity and exploration of the plural cavity is completed, under direct thoracoscopic visualization, further intercostal access is obtained. For minor procedures three 1 cm incisions are used for the corresponding “ports”, thus allowing triangulation of the instruments: the camera is usually placed in the central port and the other two are used for biopsy and retraction instruments. If open thoracotomy becomes necessary, the incisions are simply joined. At the end of the procedure, a chest tube is placed in the pleural space (36,37).
The field of VATS has evolved significantly and is now routinely performed by thoracic surgeons. The instrumentation for VATS has improved over the years. Originally, instruments used for laparoscopy were utilized for VATS. With the advancing of technology and introduction of an endoscopic linear stapler, which cuts while laying down parallel rows of staples that are hemostatic and aerostatic, has made thoracoscopic pulmonary resection a common approach (33,38). The operative time period for an undiagnosed pleural effusion is short.
Cerfolio et al., showed that under single lung ventilation in 208 patients with undiagnosed pleural effusion, the mean operative time for parietal pleural biopsy after pleural fluid drainage was 17 min (33). Because the instrumentation and video equipment continue to evolve and much of the new technology is expensive, a cost analysis for thoracoscopy compared to open thoracotomy was done by Hazelrigg and colleagues (39). The costs incurred in patients undergoing VATS wedge resection for nodules (n=45) were compared with those in similar patients having wedge resection using open techniques (n=31). Benefits, such as reduced pain, shorter operating times, and decreased hospital stays, made thoracoscopy a valuable diagnostic tool. The length of hospital stay, operating room time, disposable instrument costs, complications, and patient acuity all have an impact on the total costs and vary for different procedures.
Contraindication
Thoracoscopy is a generally well tolerated procedure. MT has an estimated mortality of 0.19-0.54% (30). There are however few contraindications to MT. Absolute contraindications to thoracoscopy are inability to tolerate partial or complete unilateral collapse of the lung, a fused pleural space with dense adhesions, shock or cardiac arrest, markedly unstable patient. Other patient factors which can make the thoracoscopic approach difficult or impossible are obesity or increased thickness of the chest wall, narrow rib spaces, a small chest or underlying conditions associated with increased bleeding (19,36,37,40).
Indication
Undiagnosed pleural effusion
When all work up for diagnosis of pleural effusion fails, thoracoscopy may be indicated. The most common two diagnosis established through thoracoscopy in such setting are malignant and tuberculous pleural effusion (41-43). Thoracoscopy should be pursued if a patient with undiagnosed pleural effusion has had at least one pleural fluid aspiration and pleural fluid cytology and adenosine deaminase (ADA) have been negative at least once (36). Hansen et al. studied 146 patients with undiagnosed pleural effusion who underwent thoracocsopy (42). The overall diagnostic sensitivity was 90.4%. The results demonstrated 62% with malignancy of the pleura and 38% revealed benign pleural diseases, among them 2% with TB. The sensitivity for malignancy was found to be 88% and the specificity 96%. The most common primary lung cancer with involvement of the pleura was the adenocarcinoma (62%), and the most common metastatic tumour originated from the breast (28%). The sensitivity for TB was 100% and the specificity 100%. No mortality was found, and the morbidity was low at about 0.6% (empyema, pleuro-cutaneous fistula, transcutaneous growth of tumour). In a study by Diacon et al., from South Africa, diagnosis of tuberculosis was made in all 42 patients included in the study (44). There are clinical clues that can help the physicians predict the likelihood of reaching a diagnosis in malignant pleural disease at thoracoscopy. In a study of 93 patients, all patient with the following 4 criteria had malignancy: 1—symptomatic period >1 month, 2—absence of fever, 3—bloody effusion, 4—chest CT scan suggestive of malignancy. Up to 25% of patients with undiagnosed exudative pleural effusion show nonspecific chronic pleuritis on thoracoscopic biopsy of the pleura. Up to 10% of these patients go on to develop malignancy, mesothelioma in particular (45,46). In our practice, we follow these patients both clinically and through imaging.
Malignant pleural effusion
As mentioned above, most published data, suggest a diagnostic yield of higher than 90% in malignant pleural effusion using thoracoscopy for pleural biopsy (41-43,46). Additionally, thoracoscopic parietal pleural biopsy in an invaluable diagnostic tool for the diagnosis of malignant pleural mesothelioma. In 1993, Boutin and colleagues published their experience on 188 patients with malignant pleural mesothelioma, 98% of whom were diagnosed on thoracoscopy (47). This is particularly important given the diagnostic yield of thoracentesis and pleural fluid cytology is even lower than that of other pleural malignancies (26-32% compared to 50-60%) (47-49). Boutin recommended port side prophylactic radiation in patients with mesothelioma, to decrease seeding of the tract with malignant cells (50). In addition to its diagnostic value, thoracoscopy can also be used for therapeutic management of malignant pleural effusion. This can be done through pleurodesis using a pleurodesing agent. There are many debates about the most appropriate method of pleurodesis in a patient with a diagnosis on malignant pleural effusion. Dresler et al., randomized 482 patients with malignant pleural effusion to talc slurry through tube thoracostomy vs. talc insufflation through thoracoscopy and showed a pleurodesis success rate of 78% and 71% respectively (51). The study’s methodology is however criticized significantly and the results remain to be speculated upon. However, in another study, Yim et al. showed that the rate of pleurodesis comparing the two techniques were not significantly different in 55 patients. In his study VATS and talc insufflation was compared to talc slurry through tube thoracostomy (52). In 2006, Debeljak et al. compared thoracoscopic talc insufflation and talc slurry through tube thoracostomy in 71 patients. Pleurodesis rate was 81% and 93% respectively and were considered equally effective. However, thoracoscopic pleurodesis was accompanied with considerably more complications (53). Richard Light suggests that it does not seem reasonable to subject a patient with a known malignant pleural effusion to general anesthesia and thoracoscopy when they could be managed just as effectively through tube thoracostomy and intrapleural tetracycline or Doxycyclin (36). In our practice, when dealing with undiagnosed malignant pleural effusion, we perform rapid onsite pathology examination of pleural biopsy specimens. If the preliminary result as well as the gross findings and the pre-test probability all suggest malignancy and in the setting of re-expandable lung, we perform talc insufflation for pleurodesis. This approach prevents the patient from undergoing two separate procedures. When talc is used as the pleurodesing agent, only graded, large particle size talc should be used. In addition to talc and tetracycline derivatives, an alternative approach is mechanical abrasion which is often done by a surgeon while performing VATS. Crnjac reported his results of mechanical abrasion through VATS vs. talc slurry and has a pleurodesis success rate of 89% and 74% respectively (54).
Finally, it is noteworthy that the performance of thoracoscopy without the use of any pleurodesing agent or mechanical abrasion has a 50% chance of pleurodesis in patients with malignant pleural effusion (55,56).
Tuberculous pleural effusion
Pleural effusion is not infrequently the first clinical manifestation of TB. Detection of ADA is highly specific for the diagnosis of tuberculous pleural effusion.
In one study of 254 patients with tuberculous pleural effusion, 253 patients had ADA level of >47 U/L (57). However, the test interpretation in the right clinical setting has been difficult and further studies have suggested the diagnosis of tuberculous pleurisy remains challenging at times (48,58). “Blind” pleural biopsy has been shown to be efficient in the diagnosis of tuberculous pleurisy and is reported to have a high diagnostic yield in parts of the world with higher prevalence of TB (44). For its inexpensiveness and availability, closed pleural biopsy seems to be an appropriate initial approach in high prevalence areas. However, MT can offer invaluable additional diagnostic ability when the diagnosis remained unclear. MT has shown to have a higher diagnostic yield (range, 91-93) than closed pleural biopsy in multiple studies (24,59). Diacon et al. compared Abram’s needle closed pleural biopsy with MT in patients with tuberculous pleurisy. He showed a diagnostic yield of 79% vs. 100% respectively (44). Additionally pooled results from of six different studies conducted in low prevalence areas showed diagnostic yield of 93.3% by MT (30).
Parapneumonic pleural effusion
In a case of an infected pleural space such as parapneumonic pleural effusion and empyema, the cornerstone of management is evacuating the pleural space from infected fluid in an efficient way. When therapeutic thoracentesis and tube thoracostomy fail and the pleural space is parted with adhesions and fibrous bands separating loculated space of fibrinopurulent fluid, one needs to look into other modalities to clear the space. Intrapleural tissue plasminogen activator and DNAase has shown to improve drainage and breaking of adhesions, decrease frequency of surgical referral and length of hospital stay (60). However, if this method fails, or in patients who are good surgical candidates with very thick and complex adhesion or those who are septic and need pleural space evacuation in a more timely fashion, thoracoscopy is indicated. During thoracoscopy, the loculated spaces cannot disrupted, completely evacuated and patient can be assessed for possible need of decortication, followed by chest tube placement. If VATS fails to completely empty the infectious debris and complete re-expansion of the lung is not achieved, the procedure is converted to open thoracotomy (61). Luh et al. reported on their management of parapneumonic effusions and empyema in 234 patients. More than 85% (200 patients) received preoperative diagnostic or therapeutic thoracentesis, tube thoracostomy, or fibrinolytics [tissue plasminogen activator (TPA) alone was used without use of DNAase] (62). Indications for VATS included empyema refractory to medical control or peel or multiloculated exudates per CT and chest tapping. Mean procedural time was 64.3±22.5 min (range, 26 to 244 min). Sixteen patients (6.8%) needed further surgery for empyema [nine patients required open drainage or thoracoplasty, and seven patients needed redecortication or repair of bronchopleural fistula (BPF)]. There were no intraoperative deaths and only eight (3.4%) perioperative deaths (<30 days), which were mostly unrelated to surgery. A total of 202 patients (86.3%) achieved satisfactory results with VATS treatment. Patients requiring open decortication or repeat procedures (40 patients) had a longer mean duration of preoperative symptoms, longer mean duration of preoperative hospitalization, and a higher ratio of pleural empyema (vs. complicated parapneumonic effusion) than patients undergoing simple VATS. It is noteworthy that the only fibrinolytics used prior to VATS were TPA. However current guidelines and recommendations, available years after Luh reported the above, suggest that this approach is no superior to intrapleural placebo. Older studies from 1990s support Luh’s findings, when VATS is used for the management of complex pleural infections (63-65). MT has limited utility in the management of complex parapneumonic effusions and empyema. In the case of thick fibrous bands and loculations that require adhesionlysis, decortication or conversion to open thoracotomy the procedure should be left to the hands of an experienced surgeon in an intubated patient under general anesthesia. Despite sparse literature in the management of complex pleural space infections and MT, a success rate of 91% is reported in one study of 127 patients with empyema who underwent MT and required no additional procedures (66). Solèr et al. reported on 16 patients with complicated parapneumonic effusion or empyema, in whom, after a failed attempt at tube drainage, MT was performed for debridement and placement of a chest tube. Definitive cure was noted in 12 of 16. In four patients open surgical debridement was necessary. In a subgroup of thoracoscopically treated patients, lung function tests were performed at least 6 months after the procedure and did not demonstrate significant restrictive changes (67).
Colt reported successful drainage of empyema in six of seven patients with only one requiring decortication. However, it is noteworthy, that the procedures were done in the operating room, while most of the patients had double lumen intubation under general anesthesia (68).
Pneumothorax
Primary spontaneous pneumothorax has a recurrence rate of 50%. The rate of recurrence increases after the first recurrence, hence thoracoscopy is recommended for patients with primary spontaneous pneumothorax in whom aspiration has failed or have a recurrent pneumothorax. Secondary spontaneous pneumothorax however has a much higher mortality and thoracoscopy is recommended after the initial drainage with tube thoracostomy (69).
Thoracoscopy is used for pleurodesis as well as for the management of bullous disease in both primary and secondary spontaneous pneumothorax. Bulla can be managed with endostapling or electrocoagulation, an older technique used much less frequently due to its resulting high recurrence rate. Mechanical abrasion of the pleura and talc insufflation have comparable results for pleurodesis, although mechanical abrasion does not carry the risk of acute respiratory failure occasionally seen with talc. Cardillo and colleagues showed a 4.4% recurrence rate in 38 months, in 432 patients who underwent VATS and endostapling. However 2.3% of the 432 required conversion to open thoracotomy (70). Another study of 483 patients with primary spontaneous pneumothorax with pleurodesis by mechanical abrasion showed a recurrence rate of 1.7% (n=66) over a 20-month period follow-up (71). Margolis et al. however showed an even superior recurrence rate of zero over a 62-month follow-up period, in their study of 156 patients who underwent VATS, stapling of blebs followed by mechanical abrasion. Patients’ mean duration of hospital stay was only 2.4 days (72).
MT has been successfully used for the management of select patient population with primary or secondary spontaneous pneumothorax. In one study from Switzerland, 108 patients were prospectively randomized to two groups of talc insufflation by MT vs. chest tube drainage (73). Patients who had larger than 5 cm bullae were excluded from this study. Recurrence rate was 5% and 34% respectively. The study also provided cost analysis among both groups and noted that cost calculation favored pleurodesis by MT. Lee and colleagues prospectively enrolled patients with severe chronic obstructive pulmonary disease (COPD) and secondary spontaneous pneumothorax who were treated with MT and talc insufflation. In their study of 41 patients, majority of spontaneous pneumothoraces measured 20% to 50% in size, and 34% were recurrent (74). Post procedure chest tube drainage and hospital stay were 4 and 5 days respectively. They reported a success rate of 95% in 35 months follow-up period. Four patients with FEV1 of less than 40% predicated died within 30 days of procedure resulting in a mortality rate of 10%. A Chinese study reported their experience on a patients with moderate to severe COPD with secondary spontaneous pneumothorax. Patients were treated by VATS of bullous disease and pleurodesis (75). They reported a mortality rate of 4.7% with 25% morbidity mostly related to persistent air leak which was treated with intrapleural injection of human fibrinogen for the treatment of postoperative persistent air leaks with success rate of 86.7%. Multivariate analysis of their postoperative complications suggested that patients with higher baseline pCO2 level were at higher risk. There are no head to head trials comparing the utility of MT and VATS in the management of spontaneous pneumothorax. There are guidelines by both British Thoracic Society (BTS) and American College of Chest Physicians (ACCP) in the management of spontaneous pneumothoraces (76,77). ACCP recommends intraoperative bullectomy and pleurodesis in recurrent primary spontaneous pneumothorax with apical bullae. BTS recommends that chemical pleurodesis be used for recurrent pneumothorax, only if the patient is unwilling to undergo surgery or is not a surgical candidate. BTS also recommended surgical intervention in the setting of recurrent pneumothorax, bilateral spontaneous pneumothorax, persistent air leak (>5-7 days of chest tube drainage or failure of complete lung re-expansion) and for professions at risk (pilots, drivers, etc.). However, BTS was not specific about which surgical intervention is preferred for primary or secondary spontaneous pneumothorax.
Hemothorax
Open thoracotomy used to be the procedure of choice for the management of hemothorax and removing of blood clots from the pleural space. It is however shown that VATS is not only an effective procedure but also cost-effective with lower incidence of complications and shorter hospital stay. In one study, 33 of 40 patients with hemothorax had successful management of their hemothorax through VATS (78). Retained blood that occupies more than 30% of hemithorax needs to be removed and is shown to be effectively done through VATS (78-81).
In 2005, Oğuzkaya et al. compared the effectiveness of VATS vs. intrapleural streptokinase and showed that VATS was significantly superior to intrapleural streptokinase (80). Although may be underreported, there is no literature on MT in hemothorax management, or its comparison with VATS in this setting.
Hepatic hydrothorax
After medical therapy has failed, the best management options for hepatic hydrothorax are liver transplant and transjugular portal systemic shunt (TIPS). The literature is sparse, when it comes to the management of hepatic hydrothorax with other modalities. VATS has been used to close diaphragmatic defects and for the purpose of pleurodesis. In one study of 18 patients, VATS and talc insufflation was performed. A total of 28% were found to have diaphragmatic defects that were closed. The procedure however was effective in 48% of patients and 3-month post procedure mortality was 30% (82). The evidence for routine VATS or MT and pleurodesis in these patients population is minimal.
Chylothorax
Thoracic duct injury is a rare but serious complication following chest surgeries and major neck dissections. One of the clinical presentations of thoracic duct injury is chylothorax. Without treatment, is up to 50% and thus, early aggressive therapy is indicated. Traditional conservative management includes low-fat diet, parenteral nutrition, careful monitoring of fluid and electrolytes, and drainage of the chylothorax. Patients with failed conservative management require definitive treatment in the form of ligation of the thoracic duct, which was traditionally done by thoracotomy. Thoracoscopic ligation of the thoracic duct has been published in 14 reports in the literature by thoracic duct ligation or clipping. Most of these reports are chylothorax in the setting of thoracic duct injury; however there are reports of successful VATS thoracic duct ligation in Lymphangioleiomyomatosis (83,84).
Postpneumonectomy empyema (PPE)
Empyema occurring after pneumonectomy is a major complication usually diagnosed during the same hospitalization. However, late-onset empyemas are not infrequent and may be difficult to diagnose. PPE successful therapy is difficult and associated with high morbidity and prolonged hospital stay. PPE is often the result of BPF or pleural infection. Schneiter et al. proposed the concept for accelerated treatment, which consists of radical debridement of the pleural cavity and packing with wet dressings of povidoneiodine. This is repeated in the operating theater every second day, until the chest cavity is macroscopically clean. If present, bronchial stump insufficiency is closed. Finally, the pleural space is obliterated with antibiotic solution (85). Gossot and colleagues reported on his experience of 11 patients who underwent thoracoscopic management of their PPE. Ten of these patients had no proven BPF. After thoracoscopic debridement of the pleural space no irrigation was done post operatively. Chest tubes were removed in 5-13 days in eight patients, with no further recurrence of empyema. Three patients required open window thoracostomy (86). There are very few other reports of VATS management of PPE but the existing reports show that VATS may be a valuable approach to the management of PPE.
Complication
MT has a favorable safety profile and when performed under local anesthesia and moderate sedation, is considered an overall safe procedure (68,87). The BTS reported 16 cases of death in 4,736 cases across 47 studies, a mortality rate of 0.3% (30). When separating diagnostic and therapeutic procedures, the authors noted that the mortality was zero in diagnostic MT (0/2,421 patients) and noted a mortality rate of 16/2,315 (0.6%) in therapeutic MT, when talc was introduced. Interestingly, 9 of 16 patients came from a single large randomized trial for talc insufflation when nongraded talc was used (51). Other major complications noted among 47 studies were separated to major and minor complications. Major complications (empyema, haemorrhage, port site tumor growth, BPF, postoperative pneumothorax or air leak and pneumonia) occurred in 86/4,736 cases (1.8%). Minor complications (subcutaneous emphysema, minor haemorrhage, operative skin site infection, hypotension during procedure, fever, atrial fibrillation) were reported in 177/2,411 procedures (7.3%). With the advent of flex-rigid scope, the rate of complications has arguably dropped. Agarwal et al., reviewed 17 studies of exudative pleural effusion undergoing MT with flex-rigid scope, a total of 755 patients. They noted major complication rate of 1.5% (11/755 patients), minor complication rate of 10.5% (79/755 patients) and no mortality (88). VATS carries a similar list of complications although both mortality and morbidity are reported to be higher. Most of the published literature however has reported on the complication rate of VATS as a thoracic procedure dealing with not only pleural diseases, but other diseases to the chest and different applications of VATS. VATS Study Group registry collected 1,820 patients among 40 participating institutions (39). They noted that lung nodules and pleural effusions were the most frequent indications, and wedge resection and operation in the pleural space were the most common procedures performed. Prolonged air leak (>5 days) was the most frequent complication and occurred in 3.2% of patients while hemorrhage requiring transfusion occurred in 1%, pneumonia and empyema occurred in 1.1% and 0.6% respectively.
In a study from France, results of VATS done in 937 patients across four institutions were reported. About one half of these patients had a pleural disease. Perioperative incidents or complications occurred in 35 patients (3.7%), and 116 procedures (12.4%) were converted to a thoracotomy. The in-hospital mortality rate was 0.5% (five patients), and death occurred principally in patients operated on for malignant pleural effusion. The overall incidence of postoperative complication was 10.9%, and the most prevalent complications were prolonged air leak (6.7%) and pleural effusion (0.7%). Majority air leaks were after wedge resection. Neurologic deficits were mostly noted after mediastinal procedures such as mediastinal tumor resection. Four patients with malignant pleural effusion died 1 month post operation and one patient with gunshot wound to chest with a bullet removed thoracoscopically from the pericardium died suddenly in 36 hours after operation. There are no head to head studies comparing MT and VATS when used in similar patient population (89).
Conclusions
Thoracoscopy is a valuable diagnostic and therapeutic tool with multiple implications in the management of the diseases of the pleura. Both MT and VATS carry acceptable safety profile with low mortality rates reported in the literature. MT is generally performed by internal medicine trained physicians, particularly interventional pulmonologists, under moderate sedation with local anesthetics in a spontaneously breathing patient and generally outside the operating room and generally requires one single port of entry to the thoracic cavity. VATS is generally performed by a surgeon under general anesthesia in an intubated patient in the operating room and requires at least three ports of entry to the thoracic cavity. Outside the realms of the pleural space, VATS has multiple other indications for other diseases of the thorax. Pleural biopsies and pleurodesis can be performed through both techniques, however, VATS allows for a more efficient drainage of loculated effusions trapped in dense fibrous bands, and most importantly, can be converted to open thoracotomy if necessary. The major advantages of MT are that it can be a cost-effective procedure in patients with poor tolerance for general anesthesia, in an outpatient setting. Ideally, every case of pleural disease requiring thoracoscopy, should be discussed between surgeon and interventional pulmonologist to tailor the most effective, safest approach for patient management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hatzinger M, Häcker A, Langbein S, et al. Hans-Christian Jacobaeus (1879-1937): The inventor of human laparoscopy and thoracoscopy. Urologe A 2006;45:1184-6. [PubMed]
- Sir Francis Cruise M.D. Br Med J 1912;1:586.
- Gordon S. Art. VIII.—Clinical reports of rare cases, occurring in the Whitworth and Hardwicke Hospitals. The Dublin Quart J Med Sci 1866;41:83-99.
- Kelling G. Ueber oesaphogoskopie, gastroskopie und kölioscopie. Münch Med Wochenschr 1902;49:21-4.
- Jacobaeus HC. Uber die möglichkeit die zystoskopie bei Untersuchung seröser höhlungen anzuwenden. Münch Med Wochenschr 1910;57:2090-2.
- Jacobaeus HC. The Cauterization of Adhesions in Artificial Pneumothorax Treatment of Pulmonary Tuberculosis under Thoracoscopic Control. Proc R Soc Med 1923;16:45-62. [PubMed]
- Thomas PA Jr. A thoracoscopic peek: what did Jacobaeus see? Ann Thorac Surg 1994;57:770-1. [PubMed]
- Loddenkemper R, Mathur PN, Noppen M, et al. Medical thoracoscopy/pleuroscopy: manual and atlas. Thieme, 2010 .
- Tassi GF, Tschopp JM. The centenary of medical thoracoscopy. Eur Respir J 2010;36:1229-31. [PubMed]
- Adler RH, Rappole BW. Recurrent malignant pleural effusions and talc powder aerosol treatment. Surgery 1967;62:1000-6. [PubMed]
- Andersen I, Poulsen T. Surgical treatment of spontaneous pneumothorax. Acta Chir Scand 1959;118:105-12. [PubMed]
- McLean AN, Bicknell SR, McAlpine LG, et al. Investigation of pleural effusion: an evaluation of the new Olympus LTF semiflexible thoracofiberscope and comparison with Abram's needle biopsy. Chest 1998;114:150-3. [PubMed]
- Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med 2007;357:2277-84. [PubMed]
- McLoud TC, Flower CD. Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol 1991;156:1145-53. [PubMed]
- Shojaee S, Argento AC. Ultrasound-guided pleural access. Semin Respir Crit Care Med 2014;35:693-705. [PubMed]
- Medford AR, Agrawal S, Bennett JA, et al. Thoracic ultrasound prior to medical thoracoscopy improves pleural access and predicts fibrous septation. Respirology 2010;15:804-8. [PubMed]
- Light RW. Thoracoscopy. Pleural diseases. sixth edition. Chapter 30. Philadelphia: Lippincott, Williams & Wilkins, 2012:481.
- Boutin C, Viallat JR, Aelony Y. Practical thoracoscopy. Berlin, Germany: Springer-Verlag, 1991.
- Marchetti G, Valsecchi A, Indellicati D, et al. Ultrasound-guided medical thoracoscopy in the absence of pleural effusion. Chest 2015;147:1008-12. [PubMed]
- Khan MA, Ambalavanan S, Thomson D, et al. A comparison of the diagnostic yield of rigid and semirigid thoracoscopes. J Bronchology Interv Pulmonol 2012;19:98-101. [PubMed]
- Rozman A, Camlek L, Marc-Malovrh M, et al. Rigid versus semi-rigid thoracoscopy for the diagnosis of pleural disease: a randomized pilot study. Respirology 2013;18:704-10. [PubMed]
- Ernst A, Hersh CP, Herth F, et al. A novel instrument for the evaluation of the pleural space: an experience in 34 patients. Chest 2002;122:1530-4. [PubMed]
- Lee P, Colt HG. Rigid and semirigid pleuroscopy: the future is bright. Respirology 2005;10:418-25. [PubMed]
- Tassi G, Marchetti G. Minithoracoscopy: a less invasive approach to thoracoscopy. Chest 2003;124:1975-7. [PubMed]
- Tassi GF, Marchetti GP, Pinelli V. Minithoracoscopy: a complementary technique for medical thoracoscopy. Respiration 2011;82:204-6. [PubMed]
- DePew ZS, Wigle D, Mullon JJ, et al. Feasibility and safety of outpatient medical thoracoscopy at a large tertiary medical center: a collaborative medical-surgical initiative. Chest 2014;146:398-405. [PubMed]
- Cohen LB, Ladas SD, Vargo JJ, et al. Sedation in digestive endoscopy: the Athens international position statements. Aliment Pharmacol Ther 2010;32:425-42. [PubMed]
- Knape JT, Adriaensen H, van Aken H, et al. Guidelines for sedation and/or analgesia by non-anaesthesiology doctors. Eur J Anaesthesiol 2007;24:563-7. [PubMed]
- Perel A. Non-anaesthesiologists should not be allowed to administer propofol for procedural sedation: a Consensus Statement of 21 European National Societies of Anaesthesia. Eur J Anaesthesiol 2011;28:580-4. [PubMed]
- Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii54-60. [PubMed]
- Astoul P, Maldonado F. Anesthetic drugs managed by pulmonologists during medical thoracoscopy: one size does not fit all! Respiration 2014;88:265-7. [PubMed]
- Grendelmeier P, Tamm M, Jahn K, et al. Propofol versus midazolam in medical thoracoscopy: a randomized, noninferiority trial. Respiration 2014;88:126-36. [PubMed]
- Cerfolio RJ, Bryant AS, Sheils TM, et al. Video-assisted thoracoscopic surgery using single-lumen endotracheal tube anesthesia. Chest 2004;126:281-5. [PubMed]
- Katlic MR, Facktor MA. Video-assisted thoracic surgery utilizing local anesthesia and sedation: 384 consecutive cases. Ann Thorac Surg 2010;90:240-5. [PubMed]
- Kendall SW, Bryan AJ, Large SR, et al. Pleural effusions: is thoracoscopy a reliable investigation? A retrospective review. Respir Med 1992;86:437-40. [PubMed]
- Light RW. Chest tubes. Pleural diseases. sixth edition. Chapter 30. Philadelphia: Lippincott, Williams & Wilkins, 2012:483.
- Stoica SC, Walker WS. Video assisted thoracoscopic surgery. Postgrad Med J 2000;76:547-50. [PubMed]
- Krasna M, Nazem A. Thoracoscopic lung resection: use of a new endoscopic linear stapler. Surg Laparosc Endosc 1991;1:248-50. [PubMed]
- Hazelrigg SR, Nunchuck SK, Landreneau RJ, et al. Cost analysis for thoracoscopy: thoracoscopic wedge resection. Ann Thorac Surg 1993;56:633-5. [PubMed]
- Dieter RA Jr, Kuzyçz GB. Complications and contraindications of thoracoscopy. Int Surg 1997;82:232-9. [PubMed]
- Menzies R, Charbonneau M. Thoracoscopy for the diagnosis of pleural disease. Ann Intern Med 1991;114:271-6. [PubMed]
- Hansen M, Faurschou P, Clementsen P. Medical thoracoscopy, results and complications in 146 patients: a retrospective study. Respir Med 1998;92:228-32. [PubMed]
- Hucker J, Bhatnagar NK, al-Jilaihawi AN, et al. Thoracoscopy in the diagnosis and management of recurrent pleural effusions. Ann Thorac Surg 1991;52:1145-7. [PubMed]
- Diacon AH, Van de Wal BW, Wyser C, et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur Respir J 2003;22:589-91. [PubMed]
- Davies HE, Nicholson JE, Rahman NM, et al. Outcome of patients with nonspecific pleuritis/fibrosis on thoracoscopic pleural biopsies. Eur J Cardiothorac Surg 2010;38:472-7. [PubMed]
- DePew ZS, Verma A, Wigle D, et al. Nonspecific pleuritis: optimal duration of follow-up. Ann Thorac Surg 2014;97:1867-71. [PubMed]
- Boutin C, Rey F, Gouvernet J, et al. Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 2: Prognosis and staging. Cancer 1993;72:394-404. [PubMed]
- Hooper C, Lee YC, Maskell N, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii4-17. [PubMed]
- Renshaw AA, Dean BR, Antman KH, et al. The role of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Chest 1997;111:106-9. [PubMed]
- Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest 1995;108:754-8. [PubMed]
- Dresler CM, Olak J, Herndon JE 2nd, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909-15. [PubMed]
- Yim AP, Chan AT, Lee TW, et al. Thoracoscopic talc insufflation versus talc slurry for symptomatic malignant pleural effusion. Ann Thorac Surg 1996;62:1655-8. [PubMed]
- Debeljak A, Kecelj P, Triller N, et al. Talc pleurodesis: comparison of talc slurry instillation with thoracoscopic talc insufflation for malignant pleural effusions. J BUON 2006;11:463-7. [PubMed]
- Crnjac A. The significance of thoracoscopic mechanical pleurodesis for the treatment of malignant pleural effusions. Wien Klin Wochenschr 2004;116 Suppl 2:28-32. [PubMed]
- Groth G, Gatzemeier U, Häussingen K, et al. Intrapleural palliative treatment of malignant pleural effusions with mitoxantrone versus placebo (pleural tube alone). Ann Oncol 1991;2:213-5. [PubMed]
- Sørensen PG, Svendsen TL, Enk B. Treatment of malignant pleural effusion with drainage, with and without instillation of talc. Eur J Respir Dis 1984;65:131-5. [PubMed]
- Valdés L, Alvarez D, San José E, et al. Tuberculous pleurisy: a study of 254 patients. Arch Intern Med 1998;158:2017-21. [PubMed]
- Gopi A, Madhavan SM, Sharma SK, et al. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest 2007;131:880-9. [PubMed]
- Wilsher ML, Veale AG. Medical thoracoscopy in the diagnosis of unexplained pleural effusion. Respirology 1998;3:77-80. [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [PubMed]
- Wurnig PN, Wittmer V, Pridun NS, et al. Video-assisted thoracic surgery for pleural empyema. Ann Thorac Surg 2006;81:309-13. [PubMed]
- Luh SP, Chou MC, Wang LS, et al. Video-assisted thoracoscopic surgery in the treatment of complicated parapneumonic effusions or empyemas: outcome of 234 patients. Chest 2005;127:1427-32. [PubMed]
- Lawrence DR, Ohri SK, Moxon RE, et al. Thoracoscopic debridement of empyema thoracis. Ann Thorac Surg 1997;64:1448-50. [PubMed]
- Cassina PC, Hauser M, Hillejan L, et al. Video-assisted thoracoscopy in the treatment of pleural empyema: stage-based management and outcome. J Thorac Cardiovasc Surg 1999;117:234-8. [PubMed]
- Striffeler H, Gugger M, Im Hof V, et al. Video-assisted thoracoscopic surgery for fibrinopurulent pleural empyema in 67 patients. Ann Thorac Surg 1998;65:319-23. [PubMed]
- Brutsche MH, Tassi GF, Györik S, et al. Treatment of sonographically stratified multiloculated thoracic empyema by medical thoracoscopy. Chest 2005;128:3303-9. [PubMed]
- Solèr M, Wyser C, Bolliger CT, et al. Treatment of early parapneumonic empyema by "medical" thoracoscopy. Schweiz Med Wochenschr 1997;127:1748-53. [PubMed]
- Colt HG. Thoracoscopy. A prospective study of safety and outcome. Chest 1995;108:324-9. [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. [PubMed]
- Cardillo G, Facciolo F, Giunti R, et al. Videothoracoscopic treatment of primary spontaneous pneumothorax: a 6-year experience. Ann Thorac Surg 2000;69:357-61; discussion 361-2. [PubMed]
- Yim AP, Liu HP. Video assisted thoracoscopic management of primary spontaneous pneumothorax. Surg Laparosc Endosc 1997;7:236-40. [PubMed]
- Margolis M, Gharagozloo F, Tempesta B, et al. Video-assisted thoracic surgical treatment of initial spontaneous pneumothorax in young patients. Ann Thorac Surg 2003;76:1661-3; discussion 1663-4.
- Tschopp JM, Boutin C, Astoul P, et al. Talcage by medical thoracoscopy for primary spontaneous pneumothorax is more cost-effective than drainage: a randomised study. Eur Respir J 2002;20:1003-9. [PubMed]
- Lee P, Yap WS, Pek WY, et al. An Audit of medical thoracoscopy and talc poudrage for pneumothorax prevention in advanced COPD. Chest 2004;125:1315-20. [PubMed]
- Zhang Y, Jiang G, Chen C, et al. Surgical management of secondary spontaneous pneumothorax in elderly patients with chronic obstructive pulmonary disease: retrospective study of 107 cases. Thorac Cardiovasc Surg 2009;57:347-52. [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [PubMed]
- Henry M, Arnold T, Harvey J, et al. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003;58 Suppl 2:ii39-52. [PubMed]
- Villavicencio RT, Aucar JA, Wall MJ Jr. Analysis of thoracoscopy in trauma. Surg Endosc 1999;13:3-9. [PubMed]
- Carrillo EH, Richardson JD. Thoracoscopy in the management of hemothorax and retained blood after trauma. Curr Opin Pulm Med 1998;4:243-6. [PubMed]
- Oğuzkaya F, Akçali Y, Bilgin M. Videothoracoscopy versus intrapleural streptokinase for management of post traumatic retained haemothorax: a retrospective study of 65 cases. Injury 2005;36:526-9. [PubMed]
- Ahmad T, Ahmed SW, Soomro NH, et al. Thoracoscopic evacuation of retained post-traumatic hemothorax. J Coll Physicians Surg Pak 2013;23:234-6. [PubMed]
- Milanez de Campos JR, Filho LO, de Campos Werebe E, et al. Thoracoscopy and talc poudrage in the management of hepatic hydrothorax. Chest 2000;118:13-7. [PubMed]
- Kumar S, Kumar A, Pawar DK. Thoracoscopic management of thoracic duct injury: Is there a place for conservatism? J Postgrad Med 2004;50:57-9. [PubMed]
- Csiszkó A, Herr G, Sz Kiss S, et al. VATS therapy of chylothorax caused by leiomyomatosis complicated with tuberous sclerosis complex. J Minim Access Surg 2013;9:84-6. [PubMed]
- Schneiter D, Cassina P, Korom S, et al. Accelerated treatment for early and late postpneumonectomy empyema. Ann Thorac Surg 2001;72:1668-72. [PubMed]
- Gossot D, Stern JB, Galetta D, et al. Thoracoscopic management of postpneumonectomy empyema. Ann Thorac Surg 2004;78:273-6. [PubMed]
- Lee P, Hsu A, Lo C, et al. Prospective evaluation of flex-rigid pleuroscopy for indeterminate pleural effusion: accuracy, safety and outcome. Respirology 2007;12:881-6. [PubMed]
- Agarwal R, Aggarwal AN, Gupta D. Diagnostic accuracy and safety of semirigid thoracoscopy in exudative pleural effusions: a meta-analysis. Chest 2013;144:1857-67. [PubMed]
- Smit HJ, Schramel FM, Sutedja TG, et al. Video-Assisted Thoracoscopy is Feasible Under Local Anesthesia. Diagn Ther Endosc 1998;4:177-82. [PubMed]


