From electrocautery, balloon dilatation, neodymium-doped:yttrium-aluminum-garnet (Nd:YAG) laser to argon plasma coagulation and cryotherapy
Introduction
The advancement in technology and miniaturization of tools and catheters has enhanced our ability to deliver therapeutics via small working channel of a bronchoscope. A variety of interventional bronchoscopic techniques are available to achieve immediate relief of central airway obstruction (CAO) with low morbidity and mortality (1). An integrated and individualized approach to the use of these complimentary modalities is required to achieve palliation at low cost and minimize hospitalization (2,3). Therapeutic bronchoscopy, both rigid and flexible, has a high technical success rate, improves dyspnea, and quality of life in malignant central airway obstruction (mCAO) with a low complication rates (4-6). Also, when combined with other modalities including surgery, radiation, or chemotherapy it may improve survival in select subgroup of patients (7,8). Likewise, these techniques are equally effective in the management of hemorrhagic complications of mCAO and immediate relief of non-malignant CAO. This has led to better patient care and outcomes in management of complex airway disorders. Physicians managing these patients should be versatile and have competency in multiple complementary modalities for optimal treatment outcomes. We provide a clinical review of use of these techniques for management of CAO.
Electrocautery
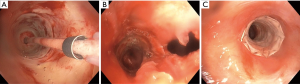
Electrocautery, often interchanged for electrosurgery (ESU), utilizes alternating high-frequency electric current passing through the probe to generate heat, which is applied to cut, coagulate, and/or vaporize tissue (9,10). There are two traditional modalities: (I) monopolar and (II) bipolar electrodes or devices. In monopolar electrodes, the most commonly used ESU modality, current flows from the generator through the active electrode, into the target tissue, through the patient, the dispersive electrode (grounding pad), and returns to the generator (11). The most common site of injury is at the patient return electrode, as such, this must be of low resistance with large enough surface area to disperse electrical current. In bipolar devices the active and return electrodes are located at the target tissue site, typically within the instrument tip. The heat generated is in direct proportion to the tissue resistance and inversely related to the vascularity and moisture content of the tissue. Electrocautery instruments commonly used with bronchoscopy include round probe, knife, wire snare, and forceps. A reusable electrocautery snare and knife is demonstrated in Figure 1A. A grounding plate, which is typically applied to the patient’s limb, allows for the electrons to leave the body when using electrocautery. The surgical effect is produced at the tip of the active electrode that due to its relatively small contact surface is the point in the circuit with the highest current density. The second electrode, i.e., the grounding plate, covers a large area discharging current while making heating here insignificant. A monopolar probe or device (snare, knife, or probe) is applied to the target after appropriate grounding to deliver energy in a controlled fashion to achieve tissue coagulation or devitalization (>60–80 °C), desiccation or drying (>100 °C), carbonization or charring (>150 °C), and vaporization or evaporation (>300 °C) based on intent and desired treatment effect (12). Its advantage is lower cost, ease of use, and wide availability in most hospitals. There are different vendors that supply ESU units and one by ConMed (Utica, NY) with pulmonary snare settings is demonstrated in Figure 1B though basic principle remains the same. The ESU combined with argon plasma coagulation (APC) units are commonly found in endoscopy units as they have applications for both gastroenterology and pulmonary in a single unit.
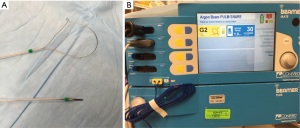
Practical aspects of setting up the system are to save pre-determined settings for different monopolar devices for commonly desired function and have all team members in-serviced on device usage. Minor variations exist for different ESU units. We recommend a power setting of 20–40 W with a blend waveform that consists of a combination of cutting and coagulation to achieve hemostasis while cutting. The newer devices though allow automated settings for desired action, e.g., cutting, snaring of tumor, tumor destruction, etc. The device will adjust for power (Watts), voltage, cutting duration and interval, making it safer for common applications in the airway. Also, if the grounding is inadequate the device will not deliver energy and show a red light indicating that troubleshooting is needed. All team members must demonstrate basic understanding of ESU and should be in-serviced in safe use of the device.
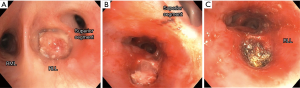
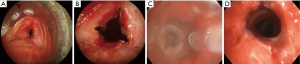
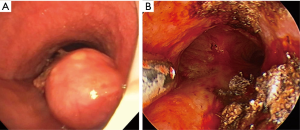
Early reports by Hopper et al. established feasibility, safety, and effectiveness of electrocautery use in restoring airway lumen in patients with predominantly mCAO (13-15). The use of EC snare device is especially suited to remove pedunculated lesions with a small stalk (Figure 2). Studies evaluating immediate response as principle outcome have found success rate ranging from 69-100% with this modality even when done under local anesthesia with mild sedation using fiberoptic bronchoscopy (16-18). In a prospective study, use of electrocautery obviated the need for laser photo-resection in majority of patients with lesions amenable to electrocautery (19) and has been demonstrated to be more cost effective compared to neodymium-doped:yttrium-aluminum-garnet (Nd:YAG) laser (20). Successful use of electrocautery as primary ablation modality for both malignant and benign CAO has been reported in a large retrospective series with a low complication rate (21). Also, radiologically occult early lung cancer in patients not eligible for surgical resection has been successfully treated with electrocautery as sole modality (22,23). Electrocautery has also been successfully used for non-malignant central airway lesions including granulation tissue related to stents, papillomas, and airway stenosis (19). Its use in making radial incisions for mucosal sparing technique of balloon dilation (BD) in web type tracheal stenosis is as effective as when done using Nd:YAG laser (24,25). Radial incisions are typically made at 12, 3, and 9 o’clock positions for mucosal sparing effect, prior to balloon or rigid dilation of airway stenosis (Figure 3). A distinct advantage of endobronchial electrocautery is its ability to coagulate at the time of its application thus minimizing the bleeding risk. This has been demonstrated in a small study where endobronchial biopsies using a hot biopsy forceps (electrocautery forceps) was associated with statistically significant reduction in bleeding score (26). The use of “hot biopsy” forceps has not become standard practice due to tissue changes and artifact (27).
Complications and precautions
Complications such as airway perforation, bronchial wall damage (27), and bleeding due to transmural perforation of airway wall into a vessel can occur. As such, meticulous attention should be drawn to the duration and power settings and it is recommended to use short bursts of energy with application limited to 1–2 s per pulse. Electrocautery consoles allow to save predetermined settings for use of different monopolar devices including snare, probe, and knife and this likely mitigates the risk of using incorrect settings and energy application.
Balloon dilatation
BD is a simple, rapid, and safe method to dilate an airway stenosis to improve luminal patency. Though initial reports described successful use of angioplasty balloon under fluoroscopic guidance for management of benign post-surgical or congenital tracheal stenosis in infants (28-30), it is now mostly performed under bronchoscopic guidance with or without other intervention techniques such as electrocautery release, laser destruction of tumor, or cryotherapy (31,32). BD results in immediate improvement in stenosis in almost all cases and offers an excellent short-term relief when used as a sole treatment modality (33). The long-term efficacy is to an extent dependent upon the pathophysiology of the underlying disease state such as in post-intubation/post-tracheotomy (34), tuberculosis (35-37), malignant (38,39), post-radiation (32), post-transplant anastomotic stenosis (40,41), sarcoidosis (32), etc. Additionally, location and complexity of the stenosis, use of adjunctive modalities, and response to treatment of underlying process such as chemotherapy and/or radiation for mCAO lesions play an important role. In most of the published large series evaluating balloon dilation in tracheobronchial stenosis, one-third to one-half of patients required other therapies including stent, laser, surgery, cryotherapy, and brachytherapy to manage symptomatic recurrences on long-term follow up (37-39,42).
BD requires appropriate equipment including balloon catheters e.g., angioplasty balloon catheter or controlled radial expansion (CRETM) balloon catheter, guide wire, and balloon insufflation device (Figure 4A-D). Wire guidance is used to bore through a high-grade stenosis or negotiate across an irregular stenosis though bronchoscopic guidance often obviates its need. Radial expanding BD is available in array of designs, lengths, and calibers. BD is made of low-compliance inflatable thermoplastic polymers that allow uniform and reproducible expansion to their specified diameter on inflation (43). Balloons are expanded by pressure injection of water or saline while hydraulic pressure of the balloon is monitored manometrically to gauge radial expansion force (Figure 4B).
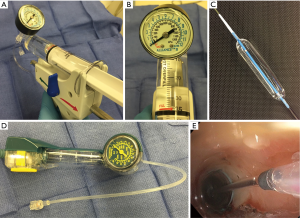
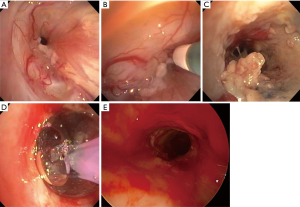
BD is performed with our without fluoroscopy, either using a guide-wire directed or direct bronchoscopic visualization method depending upon the availability of equipment and operator expertise. Fluoroscopy may be required for BD when performing a modified seldinger technique over a guide wire. The balloon catheters have radiopaque markers at the proximal and distal ends of the balloon for fluoroscopic visualization. Our preferred method is to use a CRETM balloon catheter via a 2.8 mm or larger working channel of bronchoscope or a rigid bronchoscope. This provides direct visualization and maximal control of the balloon catheter while maintaining oxygenation and ventilation pre-/post-dilation. Also, it provides ability to assess mucosal change and visualization of distal lumen as the bronchoscope is advanced on to the transparent inflated balloon while maintaining traction on the proximal end of balloon catheter and counter-traction with the scope (Figure 3C,4E,5A). It is critical to select an appropriate size of the balloon, both length and inflatable diameter, as too small of a length will result in slipping of the balloon and too long of a catheter could potentially result in distal airway trauma. Bronchoscopic estimation of the airway caliber proximal to stenosis usually provides a reasonable estimate of the desired post-dilation diameter though one must carefully assess tissue integrity and distensibility of the stenotic airway wall to minimize injury. The inflation times vary from 30 to 120 s and vary with clinical tolerance of the patient, achievement of desired objectives, and operator preferences. In general a graded and incremental dilation with repeated inflation/deflation cycles progressing to desired luminal diameter is recommended. This allows operator to assess tissue distensibility and elasticity to interplay and likely prevent airway tears.
Complications and precautions
BD is a relatively safe procedure. Even so, one must be aware of potential complications including mucosal, deep mucosal, and transmural tear (44,45) (Figure 5B), bleeding, pneumomediastinum, and pneumothorax (39,42,46). Mild amount of mucosal bleed is usually observed after balloon dilation and often does not require any intervention. A small airway wall tear and/or resultant symptomatic pneumomediastinum may be managed by placement of a covered metal stent (Figure 5C) and/or surgical correction.
Nd-YAG: laser
Laser has been particularly successful in treating tumors obstructing central airways and benign airway stenosis (47-52). Unlike electrocautery, it delivers a non-contact light energy via a catheter. Its ability to photocoagulate/vaporize/cut obstructing tissue before mechanical debulking or excision (Figure 6A,B) potentially allows for improved control of bleeding in the airway during bronchoscopy (49,53). While there are several forms of lasers, the property of photocoagulation/vaporization/cutting is based primarily on the wavelength of the laser. The Nd:YAG laser is the best described laser for use with bronchoscopy, which emits light at a wavelength of 1,064 nm and has balanced properties in ability to photo coagulate or vaporize tumor and cut stenotic lesions (54). See Table 1 for comparison of different types of lasers and its effect on tissue. Laser application is performed with either a rigid or flexible bronchoscope (50). Rigid bronchoscopy is preferred by some authors as it provides wide operating channel, the patient can be ventilated, blood and secretions aspirated, and laser coagulation utilized (49,53). However, flexible bronchoscope allows easier access to the more distal airways that may require greater angulation. The optimal delivery of the laser is primarily determined by the distance of using the laser/tissue absorption of the laser and anatomic location (54,55).

Full table
Initial reports of effectiveness of laser bronchoscopy in achieving relief of obstruction from mCAO and non-malignant CAO in very large series established credence of this modality in achieving excellent palliation (49,53). Lesions most amenable to laser therapy are central, intrinsic, short (<4 cm), with a visible distal endobronchial lumen. When lesions meet these criteria, potency can be res-established in more than 90% of cases (1,51,52,56,57). Combination therapy with radiation has shown to prolong the relief of symptoms and lessen disease progression (58,59). Further, patients undergoing multi-modality treatment with laser and brachytherapy may have improved survival compared to laser treatment alone (7,60). This may be attributable to relief of airway obstruction and improved performance status and patient’s ability to undergo multimodality treatment. Additionally, endobronchial laser has been reported to have successfully used in destruction of broncholith (61), resection of post-transplant granulation tissue (62), endobronchial amyloidosis (63) and endobronchial endometriosis (64).
One disadvantage of Nd:YAG laser is its high cost and possible alternatives are APC and cryotherapy systems. However, lasers offer a deeper penetration, non-contact method of delivery, and offer a balance photocoagulation/vaporization. Comparative depth of penetration of different ablative modalities is summarized in Table 2. Similar to electrocautery/APC, airway fire is a risk and a decreased FiO2 should be performed during its active use (56,65).

Full table
Complications and precautions
Safety of Nd:YAG laser in the airway procedures has been well established. Even so, particular attention should be directed to keep power settings less than 40 W, pulse duration of 0.5 to 1 s, and should always be aimed parallel to the airway to avoid perforation. Significant complications developed in fewer than 5% of case, and in summary of close to 7,000 laser treatment the overall complication rate was 0.99% (1). Reported complications include a very low incidence of bleeding, pneumothorax, pneumomediastinum, or death (57). The mortality has been attributed to systemic air embolism as a result of high flow of air coolant and contact probes. It is recommended that non-contact mode be used whenever possible while keeping the coaxial coolant air flow at minimum level (66).
Argon plasma coagulation (APC)
APC is an application of gas discharges in argon in ESU (67). It offers the simplicity and low cost of an electrocoagulator with the non-contact approach of an Nd:YAG laser. The major application fields are hemostasis, tissue devitalization, and tissue destruction. It was first introduced in open surgery (68) and later adapted for use in endoscopy (69,70), and its effective use in airway was first described for treatment of hemoptysis and neoplastic airway obstruction (71-73). Due to the superficial effect and need to remove eschar over treated tissue for additional devitalization, APC is often used as part of a multi-modality approach for tumor debulking (6,74,75). Benign tumors including leiomyomas, hamartomas, pleomorphic adenomas, granular cell tumors, and carcinoids have also been treated with APC alone or in combination (76,77). Non-malignant disease states such as granulation tissue associated with airway stents and surgical anastomosis along with respiratory papillomas have been successfully managed with APC (78-81). For endobronchial vascular lesions, APC has been shown to be highly effective. These include hemangiomas, fibrosing mediastinitis, and Dieulafoy lesions (82-85).
An APC delivery system is composed of an argon gas cylinder, a computer controlled high-frequency electrosurgical generator with a gas-flow controlling valve, and an endoscopic probe. This is typically mounted on the ESU unit as a combination unit (Figure 1B) for ease of use and functionality. Similar to electrocautery, a grounding plate is required for the electrons current to leave the body. Unlike electrocautery, it is a non-contact modality using the principle of ionized gas to deliver current to the desired tissue. Inert argon gas is converted to ionized argon gas, plasma, via an electrode contained within the probe. Once gas flow, electrical energy, mode, and pulse time are selected on the console, the device is controlled during the procedure with a foot pedal switch. Increased resistance created by coagulation and desiccation of the tissue limits the depth of penetration. This makes APC ideal for hemostasis and reduces the risk of injury to vital mediastinal tissues, conversely, this limits its energy penetration depth for cauterization and destruction. The cauterization and devitalization effect of APC along with balloon dilation is demonstrated in Figure 7. Additionally, as the plasma is ignited at the tip of the probe, it seeks the nearest electro-conductive area. This allows for tangential treatment of lesions or around bends where head-on alignment of the probe with the lesion is not attainable.
Practical aspects of system setup include power (watts), gas flow rate, and mode of energy delivery. Increased power results in more rapid devitalization of tissue and deeper penetration, but can increase the risk of perforation. Gas flow should be set at the lowest possible rate for desired tissue effect in order to reduce the risk of gas embolization. The mode can be set to either forced or pulsed. Forced mode entails continuous delivery of energy resulting in more rapid tissue devitalization and hemostasis. In contrast, pulsed mode sends intermittent bursts of energy to the tissue, resulting in a more superficial effect. In either mode, subtle movement of the probe in a “painting” motion with the catheter extending approximately one centimeter from the bronchoscope to avoid accidental damage is advocated. Our preferred method and initial settings are forced coagulation mode with the lowest power and flow rate to achieve the desired effect. Due to minor differences in probe characteristics, we recommend 30 W-0.8 L/min for ERBE (Germany) and 25 W-0.4 L/min for ConMed (Utica, NY) systems. Above settings are displayed on the respective APC consoles along with examples of individual probes (Figure 8A-E). Non-contact nature of energy delivery to target tissue using an ERBE probe is also illustrated (Figure 8C).

Complications and precautions
APC has a high safety profile, however, deaths from intracardiac gas embolism resulting in cardiopulmonary arrest (86), and cerebral gas embolism (87) have been reported when the gas flow rate exceeded 1-2 L/min. In animal models, gas emboli are noted even at lower flow rates of 0.5 L/min with the power set at 20 W (88). In clinical practice this can be avoided by keeping the flow to less than 0.8 L/min. Airway perforation has been described at a rate of 1.4% in a large series of patients undergoing APC (71). This risk can be minimized by keeping the applications short (1-2 s), using the above recommended flow settings, minimizing the power, and maintaining a safe distance of 2-5 mm between probe and tissue.
General precautions during use of heat energy in the airway
All types of heat energy use in the airway require airway fire safety protocols to be implemented and followed at an individual institution. Generation of an airway fire requires three items: (I) an oxidizer (oxygen or nitrous oxide); (II) an ignition source (electrocautery, laser, APC); and (III) a fuel (stents, endotracheal tube, bronchoscope). During therapeutic bronchoscopy with thermal energy, the ignition source comes in contact with the oxidizer rich environment and may result in airway fire. As such, it is imperative that FiO2 be decreased to <40% during these procedures, avoid using near ETT or covered metal and silicone stents, and use short bursts of energy at appropriate device settings (89,90). In patients with implanted permanent pacemakers or cardioverters caution must be exercised with use of electrocautery or APC and interrogation of cardiac devices should be performed if any intra-procedural event occurs. One should also avoid placement of the grounding pad on the skin over metallic prosthesis to prevent energy discharge into the prosthesis and resultant burn injury (90). Additional safety precautions are required when laser is in use including use safety glasses by all personnel and placement of placards outside the procedure room to avoid inadvertent injury to health care professionals (90,91). To enhance patient safety, one must have knowledge of institution fire safety protocols, perform fire safety drills and simulation training, assess high risk situation and facilitate team discussion, minimize or avoid oxidizer and safely manage ignition sources and potential fuel (89).
Cryotherapy
Cryotherapy is based on cytotoxic effects of cold on living tissue. The description of destroying tumors using low temperatures was first detailed in 1851 by Arnott (92), though it was not until a century later when its therapeutic effects in malignant lesions were reported (93). The application of cold energy employs the principle of Joule-Thompson effect; rapid cooling of gas upon sudden expansion from high to a low pressure (94). Application of cold energy to tissue, with repeated freeze thaw cycle, results in appearance of intra and extra-cellular ice crystals and resultant damage to intracellular organelles, in particular mitochondria (94,95). Cytodestruction varies according to rapidity of freeze thaw cycle, distance from center of application, and vascularity of tumor (96). Collagen, cartilage, or poorly vascularized tissues are relatively cryoresistant. As such, the extracellular matrix of tissue is preserved and explains healing with minimal scarring (97).
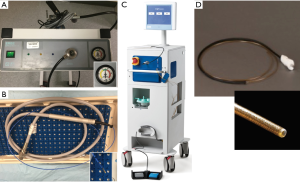
Currently, the methods of cryogen delivery that are commercially available in the USA and most suited for use in airway include: (I) reusable contact cryoprobe (rigid and flexible) with a probe tip cooled by gas decompression in the probe head using nitrous oxide (N2O) and an exit temperature of −89.5 °C (ERBE, Germany); (II) Spray Cryo with a catheter tip facilitating delivery of gaseous liquid nitrogen (N2) generating a spray and an exit temperature of −196 °C (CSA medical, Baltimore, MD). Cryotherapy units consist of a console that regulates flow of cryogen, typically via a foot pedal. A cryotherapy unit with pressurized N2O gas tank is shown in Figure 9A. Flexible cryoprobes come in two sizes, 1.9 mm (Figure 9B) and 2.4 mm, for use with minimal working channel diameter of 2.0 and 2.8 mm respectively. Rigid cryoprobes are larger and may allow for rapid thawing and resultant shorter procedure time. Spray cryotherapy unit (Figure 9C) has similar console except N2 release is more controlled and either active or appropriate passive ventilation is required during the procedure. A 7-Fr catheter (Figure 9D) that requires a minimum of 2.8 mm working channel is used to deliver the cryogen. It is currently approved in USA by the Food and Drug Administration (FDA) as a cryosurgical tool in the fields of dermatology, gynecology, and general surgery to ablate benign and malignant lesions.
The introduction of closed tip cryoprobe by Cooper and Lee paved path for local tumor destruction in different anatomic sites (93). Subsequently others reported their successes in management of refractory airway strictures (98,99) and offer palliation in malignant strictures of tracheobronchial tree (100,101). Experience of Homasson et al. with cryoprobe ablation in a large series of patients with predominantly malignant airway strictures via a rigid bronchoscope (96,101) led to the development of prototype for a more flexible, longer, and smaller tip cryoprobe that is currently in use via flexible bronchoscopy for distal tumors or strictures. Cryotherapy has been highly successful for treatment of granulation tissue (Figure 10) (Figure 10A,F) in particular post-transplantation anastomosis (102-104), tracheostomy and stent related granulation tissue (105,106). When managing stent related granulation tissue, it offers a distinct advantage over other modalities such as electrocautery, APC, or Nd:YAG laser as there is no risk of airway fire. Also, cryotherapy is very effective in removal of foreign body with high water content including blood clots (Figure 10C), aspirated food material (Figure 10D), mucus plugs, endobronchial Aspergilloma (Figure 10E), and even a chewing gum (107-109). Its effectiveness in benign trachea-bronchial lesions including lipoma, endobronchial polyp, and bronchial amyloidosis has been reported as well (110). Spray cryotherapy has limited safety/efficacy data as this a newer application of cryotherapy (111,112).

Cytodestruction of tumor is achieved by rapid freezing of the cryoprobe or application of spray for instantaneous freeze with higher efficacy being suggested by several overlapping short freezing spots in tumoral tissue (96). As the effects of cryotherapy are delayed, unless cryorecanalization using cryoadhesion is performed, additional modality is usually required to achieve rapid relief from tumor causing mCAO. Cryotherapy has been demonstrated to achieve effective palliation in patients with inoperable cancer and bronchial obstruction with improvement in symptoms, quality of life, and Karnofsky performance scores (113-116). Applying principles of cryoadhesion, cryorecanalization (Figure 10H,I) has been successfully performed for rapid relief from tumor obstruction (74,75). This may require a complimentary modality such as APC to achieve hemostasis for bleeding at the site of tumor base (75). Additionally, cryotherapy has been used in laser-assisted mechanical resection for treating the residual tumor (94,117) and in early superficial bronchogenic carcinoma where a close bronchoscopic and radiologic surveillance is advocated given a high local recurrence rate (118). Limited data of cryotherapy in conduction with radiation and chemotherapy has been promising though larger controlled studies are needed to further support this rationale (119-121).
The cryodestructive role of cryotherapy in benign and malignant lesions is well established; however its role in enhancing diagnostic yield is evolving. Cryobiopsy of endobronchial lesions (Figure 10G,H) has been shown to be safe and increases the diagnostic yield when compared with conventional forceps biopsy (122,123). Its safety and feasibility has been demonstrated in small series for diagnosis of interstitial lung disease (124,125), lung transplant surveillance (126,127), and immunocompromised patients with pulmonary infiltrates (128). Likewise, it has been feasible to obtain cryobiopsies via guide-sheath for peripheral lung nodules under endobronchial ultrasound guidance using a 1.2 mm cryoprobe (129). Thus, transbronchial cryobiopsy is a promising technique though additional large studies need to validate its safety and diagnostic yield in diffuse parenchymal lung disease (130).
Complications and precautions
Contact cryotherapy has a high safety profile. At the recommended energy delivery the collagen network is preserved and the bronchial cartilage is relatively cryoresistant thus minimizing the risk of tissue necrosis and perforation. Transient fever following cryotherapy has been observed and may be associated with cell necrosis (94). Airway sloughing of necrotic material can be abundant and obstruct the lumen, often requiring “toilet” bronchoscopy few days after cryotherapy. Bleeding following cryotherapy can be easily controlled by application of APC emphasizing possible need for multi-modality approach. Reported complications with spray cryotherapy include barotrauma, intra-operative deaths, and concerns of gas embolism (131).
Conclusions
Ablative techniques used during bronchoscopy offer a safe and effective modality for the treatment of CAO. Competency in multiple ablative modalities may provide optimal patient outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97. [PubMed]
- Mathisen DJ, Grillo HC. Endoscopic relief of malignant airway obstruction. Ann Thorac Surg 1989;48:469-73; discussion 473-5. [PubMed]
- Chan AL, Yoneda KY, Allen RP, et al. Advances in the management of endobronchial lung malignancies. Curr Opin Pulm Med 2003;9:301-8. [PubMed]
- Colt HG, Harrell JH. Therapeutic rigid bronchoscopy allows level of care changes in patients with acute respiratory failure from central airways obstruction. Chest 1997;112:202-6. [PubMed]
- Ost DE, Ernst A, Grosu HB, et al. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest 2015;147:1282-98. [PubMed]
- Ost DE, Ernst A, Grosu HB, et al. Complications Following Therapeutic Bronchoscopy for Malignant Central Airway Obstruction: Results of the AQuIRE Registry. Chest 2015;148:450-71. [PubMed]
- Shea JM, Allen RP, Tharratt RS, et al. Survival of patients undergoing Nd:YAG laser therapy compared with Nd:YAG laser therapy and brachytherapy for malignant airway disease. Chest 1993;103:1028-31. [PubMed]
- Santos RS, Raftopoulos Y, Keenan RJ, et al. Bronchoscopic palliation of primary lung cancer: single or multimodality therapy? Surg Endosc 2004;18:931-6. [PubMed]
- Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J 2006;27:1258-71. [PubMed]
- Barlow DE. Endoscopic applications of electrosurgery: a review of basic principles. Gastrointest Endosc 1982;28:73-6. [PubMed]
- Covidien/Medtronic. Principles of electrosurgery. 2014; 1-37.
- Hoffman J. Thermal effects on biological tissues. In: Berlien HP, Müller GJ, editors. Applied Laser Medicine. Berlin: Springer; 2003.
- Hooper RG, Spratling L, Beechler C, et al. Endobronchial electrocautery. A role in bronchogenic carcinoma? Endoscopy 1984;16:67-70. [PubMed]
- Hooper RG, Jackson FN. Endobronchial electrocautery. Chest 1985;87:712-4. [PubMed]
- Hooper RG, Jackson FN. Endobronchial electrocautery. Chest 1988;94:595-8. [PubMed]
- Sutedja G, van Kralingen K, Schramel FM, et al. Fibreoptic bronchoscopic electrosurgery under local anaesthesia for rapid palliation in patients with central airway malignancies: a preliminary report. Thorax 1994;49:1243-6. [PubMed]
- Sagawa M, Sato M, Takahashi H, et al. Electrosurgery with a fiberoptic bronchoscope and a snare for endotracheal/endobronchial tumors. J Thorac Cardiovasc Surg 1998;116:177-9. [PubMed]
- Sagawa M, Inoue K, Sato M, et al. Successful resection of endotracheal papillary adenocarcinoma by endoscopic electrosurgery using a new snare: report of a case. Surg Today 1999;29:570-2. [PubMed]
- Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest 2000;118:516-21. [PubMed]
- Boxem TV, Muller M, Venmans B, et al. Nd-YAG laser vs bronchoscopic electrocautery for palliation of symptomatic airway obstruction: a cost-effectiveness study. Chest 1999;116:1108-12. [PubMed]
- Wahidi MM, Unroe MA, Adlakha N, et al. The use of electrocautery as the primary ablation modality for malignant and benign airway obstruction. J Thorac Oncol 2011;6:1516-20. [PubMed]
- van Boxem TJ, Venmans BJ, Schramel FM, et al. Radiographically occult lung cancer treated with fibreoptic bronchoscopic electrocautery: a pilot study of a simple and inexpensive technique. Eur Respir J 1998;11:169-72. [PubMed]
- Vonk-Noordegraaf A, Postmus PE, Sutedja TG. Bronchoscopic treatment of patients with intraluminal microinvasive radiographically occult lung cancer not eligible for surgical resection: a follow-up study. Lung Cancer 2003;39:49-53. [PubMed]
- Tremblay A, Coulter DT, Mehta AC. Modification of a mucosal-sparing technique using electrocautery and balloon dilatation in the endoscopic management of a web-like benign airway stenosis. J Bronchol 2003;10:268-71.
- Mehta AC, Lee FY, Cordasco EM, et al. Concentric tracheal and subglottic stenosis. Management using the Nd-YAG laser for mucosal sparing followed by gentle dilatation. Chest 1993;104:673-7. [PubMed]
- Tremblay A, Michaud G, Urbanski SJ. Hot biopsy forceps in the diagnosis of endobronchial lesions. Eur Respir J 2007;29:108-11. [PubMed]
- van Boxem TJ, Westerga J, Venmans BJ, et al. Tissue effects of bronchoscopic electrocautery: bronchoscopic appearance and histologic changes of bronchial wall after electrocautery. Chest 2000;117:887-91. [PubMed]
- Cohen MD, Weber TR, Rao CC. Balloon dilatation of tracheal and bronchial stenosis. AJR Am J Roentgenol 1984;142:477-8. [PubMed]
- Brown SB, Hedlund GL, Glasier CM, et al. Tracheobronchial stenosis in infants: successful balloon dilation therapy. Radiology 1987;164:475-8. [PubMed]
- Polonovski JM, Hertz Panier L, Francois M, et al. Balloon dilatation of tracheobronchial stenoses in children. Apropos of 4 cases. Ann Otolaryngol Chir Cervicofac 1991;108:411-6. [PubMed]
- Sheski FD, Mathur PN. Long-term results of fiberoptic bronchoscopic balloon dilation in the management of benign tracheobronchial stenosis. Chest 1998;114:796-800. [PubMed]
- Chhajed PN, Malouf MA, Glanville AR. Bronchoscopic dilatation in the management of benign (non-transplant) tracheobronchial stenosis. Intern Med J 2001;31:512-6. [PubMed]
- Mayse ML, Greenheck J, Friedman M, et al. Successful bronchoscopic balloon dilation of nonmalignant tracheobronchial obstruction without fluoroscopy. Chest 2004;126:634-7. [PubMed]
- Zias N, Chroneou A, Tabba MK, et al. Post tracheostomy and post intubation tracheal stenosis: report of 31 cases and review of the literature. BMC Pulm Med 2008;8:18. [PubMed]
- Nakamura K, Terada N, Ohi M, et al. Tuberculous bronchial stenosis: treatment with balloon bronchoplasty. AJR Am J Roentgenol 1991;157:1187-8. [PubMed]
- Low SY, Hsu A, Eng P. Interventional bronchoscopy for tuberculous tracheobronchial stenosis. Eur Respir J 2004;24:345-7. [PubMed]
- Cho YC, Kim JH, Park JH, et al. Tuberculous Tracheobronchial Strictures Treated with Balloon Dilation: A Single-Center Experience in 113 Patients during a 17-year Period. Radiology 2015;277:286-93. [PubMed]
- Lee KH, Ko GY, Song HY, et al. Benign tracheobronchial stenoses: long-term clinical experience with balloon dilation. J Vasc Interv Radiol 2002;13:909-14. [PubMed]
- Hautmann H, Gamarra F, Pfeifer KJ, et al. Fiberoptic bronchoscopic balloon dilatation in malignant tracheobronchial disease: indications and results. Chest 2001;120:43-9. [PubMed]
- Keller C, Frost A. Fiberoptic bronchoplasty. Description of a simple adjunct technique for the management of bronchial stenosis following lung transplantation. Chest 1992;102:995-8. [PubMed]
- De Gracia J, Culebras M, Alvarez A, et al. Bronchoscopic balloon dilatation in the management of bronchial stenosis following lung transplantation. Respir Med 2007;101:27-33. [PubMed]
- Noppen M, Meysman M, D'Haese J, et al. Interventional bronchoscopy: 5-year experience at the Academic Hospital of the Vrije Universiteit Brussel (AZ-VUB). Acta Clin Belg 1997;52:371-80. [PubMed]
- ASGE Technology Committee, Siddiqui UD, Banerjee S, et al. Tools for endoscopic stricture dilation. Gastrointest Endosc 2013;78:391-404. [PubMed]
- Kim JH, Shin JH, Song HY, et al. Tracheobronchial laceration after balloon dilation for benign strictures: incidence and clinical significance. Chest 2007;131:1114-7. [PubMed]
- Kim JH, Shin JH, Shim TS, et al. Deep tracheal laceration after balloon dilation for benign tracheobronchial stenosis: case reports of two patients. Br J Radiol 2006;79:529-35. [PubMed]
- MCArdle JR, Gildea TR, Mehta AC. Balloon bronchoplasty. Its indications, benefits, and complications. J Bronchol 2005;12:123-7.
- Laforet EG, Berger RL, Vaughan CW. Carcinoma obstructing the trachea. Treatment by laser resection. N Engl J Med 1976;294:941. [PubMed]
- Dumon JF, Reboud E, Garbe L, et al. Treatment of tracheobronchial lesions by laser photoresection. Chest 1982;81:278-84. [PubMed]
- Dumon JF, Shapshay S, Bourcereau J, et al. Principles for safety in application of neodymium-YAG laser in bronchology. Chest 1984;86:163-8. [PubMed]
- Toty L, Personne C, Colchen A, et al. Bronchoscopic management of tracheal lesions using the neodynium yttrium aluminium garnet laser. Thorax 1981;36:175-8. [PubMed]
- Cavaliere S, Venuta F, Foccoli P, et al. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest 1996;110:1536-42. [PubMed]
- Personne C, Colchen A, Leroy M, et al. Indications and technique for endoscopic laser resections in bronchology. A critical analysis based upon 2,284 resections. J Thorac Cardiovasc Surg 1986;91:710-5. [PubMed]
- Cavaliere S, Foccoli P, Toninelli C, et al. Nd-YAG laser therapy in lung cancer: An 11 year experience with 2253 applications in 1585 patients. J Bronchol 1994;1:105-11.
- Lee HJ, Malhotra R, Grossman C, et al. Initial Report of Neodymium: Yttrium-Aluminum-Perovskite (Nd: YAP) Laser Use During Bronchoscopy. J Bronchology Interv Pulmonol 2011;18:229-32. [PubMed]
- Chan AL, Tharratt RS, Siefkin AD, et al. Nd:YAG laser bronchoscopy. Rigid or fiberoptic mode? Chest 1990;98:271-5. [PubMed]
- Kvale PA, Eichenhorn MS, Radke JR, et al. YAG laser photoresection of lesions obstructing the central airways. Chest 1985;87:283-8. [PubMed]
- Cavaliere S, Foccoli P, Farina PL. Nd:YAG laser bronchoscopy. A five-year experience with 1,396 applications in 1,000 patients. Chest 1988;94:15-21. [PubMed]
- Macha HN, Becker KO, Kemmer HP. Pattern of failure and survival in endobronchial laser resection. A matched pair study. Chest 1994;105:1668-72. [PubMed]
- Chella A, Ambrogi MC, Ribechini A, et al. Combined Nd-YAG laser/HDR brachytherapy versus Nd-YAG laser only in malignant central airway involvement: a prospective randomized study. Lung Cancer 2000;27:169-75. [PubMed]
- Han CC, Prasetyo D, Wright GM. Endobronchial palliation using Nd:YAG laser is associated with improved survival when combined with multimodal adjuvant treatments. J Thorac Oncol 2007;2:59-64. [PubMed]
- Miks VM, Kvale PA, Riddle JM, et al. Broncholith removal using the YAG laser. Chest 1986;90:295-7. [PubMed]
- Madden BP, Kumar P, Sayer R, et al. Successful resection of obstructing airway granulation tissue following lung transplantation using endobronchial laser (Nd:YAG) therapy. Eur J Cardiothorac Surg 1997;12:480-5. [PubMed]
- Madden BP, Lee M, Paruchuru P. Successful treatment of endobronchial amyloidosis using Nd:YAG laser therapy as an alternative to lobectomy. Monaldi Arch Chest Dis 2001;56:27-9. [PubMed]
- Puma F, Carloni A, Casucci G, et al. Successful endoscopic Nd-YAG laser treatment of endobronchial endometriosis. Chest 2003;124:1168-70. [PubMed]
- Casey KR, Fairfax WR, Smith SJ, et al. Intratracheal fire ignited by the Nd-YAG laser during treatment of tracheal stenosis. Chest 1983;84:295-6. [PubMed]
- Tellides G, Ugurlu BS, Kim RW, et al. Pathogenesis of systemic air embolism during bronchoscopic Nd:YAG laser operations. Ann Thorac Surg 1998;65:930-4. [PubMed]
- Morrison Jr CF, inventor; Valleylab Inc., assignee. Electrosurgical method and apparatus for initiating an electrical discharge in an inert gas flow. United States patent 4040426, 1977.
- Quinlan DM, Naslund MJ, Brendler CB. Application of argon beam coagulation in urological surgery. J Urol 1992;147:410-2. [PubMed]
- Farin G, Grund KE. Technology of argon plasma coagulation with particular regard to endoscopic applications. Endosc Surg Allied Technol 1994;2:71-7. [PubMed]
- Grund KE, Storek D, Farin G. Endoscopic argon plasma coagulation (APC) first clinical experiences in flexible endoscopy. Endosc Surg Allied Technol 1994;2:42-6. [PubMed]
- Reichle G, Freitag L, Kullmann HJ, et al. Experience with Argon Plasma Coagulation (APC) in bronchology. A report of clinical experience after 482 applications. J Bronchol 2000;7:109-17.
- Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001;119:781-7. [PubMed]
- Crosta C, Spaggiari L, De Stefano A, et al. Endoscopic argon plasma coagulation for palliative treatment of malignant airway obstructions: early results in 47 cases. Lung Cancer 2001;33:75-80. [PubMed]
- Schumann C, Hetzel M, Babiak AJ, et al. Endobronchial tumor debulking with a flexible cryoprobe for immediate treatment of malignant stenosis. J Thorac Cardiovasc Surg 2010;139:997-1000. [PubMed]
- Hetzel M, Hetzel J, Schumann C, et al. Cryorecanalization: a new approach for the immediate management of acute airway obstruction. J Thorac Cardiovasc Surg 2004;127:1427-31. [PubMed]
- Miller SM, Bellinger CR, Chatterjee A. Argon plasma coagulation and electrosurgery for benign endobronchial tumors. J Bronchology Interv Pulmonol 2013;20:38-40. [PubMed]
- Jabbardarjani H, Masjedi M, Herth F. Successful treatment of endobronchial carcinoid using argon plasma coagulation. J Bronchology Interv Pulmonol 2009;16:196-8. [PubMed]
- Keller CA, Hinerman R, Singh A, et al. The use of endoscopic argon plasma coagulation in airway complications after solid organ transplantation. Chest 2001;119:1968-75. [PubMed]
- Colt HG. Bronchoscopic resection of Wallstent-associated granulation tissue using argon plasma coagulation. J Bronchol 1998;5:209-212.
- Sato M, Terada Y, Nakagawa T, et al. Successful use of argon plasma coagulation and tranilast to treat granulation tissue obstructing the airway after tracheal anastomosis. Chest 2000;118:1829-31. [PubMed]
- Bergler W, Hönig M, Götte K, et al. Treatment of recurrent respiratory papillomatosis with argon plasma coagulation. J Laryngol Otol 1997;111:381-4. [PubMed]
- deKeratry DR. Argon Plasma Coagulation for endobronchial hemangioma: a new treatment option for a rare cause of hemoptysis. J Bronchol 2004;11:254-6.
- Rose AS, Mathur PN. Endobronchial capillary hemangioma: case report and review of the literature. Respiration 2008;76:221-4. [PubMed]
- Manali ED, Saad CP, Krizmanich G, et al. Endobronchial findings of fibrosing mediastinitis. Respir Care 2003;48:1038-42. [PubMed]
- Dalar L, Sökücü SN, Özdemir C, et al. Endobronchial argon plasma coagulation for treatment of Dieulafoy disease. Respir Care 2015;60:e11-3. [PubMed]
- Reddy C, Majid A, Michaud G, et al. Gas embolism following bronchoscopic argon plasma coagulation: a case series. Chest 2008;134:1066-9. [PubMed]
- Shaw Y, Yoneda KY, Chan AL. Cerebral gas embolism from bronchoscopic argon plasma coagulation: a case report. Respiration 2012;83:267-70. [PubMed]
- Feller-Kopman D, Lukanich JM, Shapira G, et al. Gas flow during bronchoscopic ablation therapy causes gas emboli to the heart: a comparative animal study. Chest 2008;133:892-6. [PubMed]
- Apfelbaum JL, Caplan RA, Barker SJ, et al. Practice advisory for the prevention and management of operating room fires: an updated report by the American Society of Anesthesiologists Task Force on Operating Room Fires. Anesthesiology 2013;118:271-90. [PubMed]
- Mahmood K, Wahidi MM. Ablative therapies for central airway obstruction. Semin Respir Crit Care Med 2014;35:681-92. [PubMed]
- Du Rand IA, Barber PV, Goldring J, et al. British Thoracic Society guideline for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax 2011;66 Suppl 3:iii1-21. [PubMed]
- Arnott J. On the treatment of cancer through the regulated application of an anaesthetic temperature. London: Churchill, 1851; 32.
- Cooper IS, Lee AS. Cryostatic congelation: a system for producing a limited, controlled region of cooling or freezing of biologic tissues. J Nerv Ment Dis 1961;133:259-63. [PubMed]
- Vergnon JM, Huber RM, Moghissi K. Place of cryotherapy, brachytherapy and photodynamic therapy in therapeutic bronchoscopy of lung cancers. Eur Respir J 2006;28:200-18. [PubMed]
- Mazur P. Cryobiology: the freezing of biological systems. Science. 1970;168:939-49. [PubMed]
- Homasson JP, Thiery JP, Angebault M, et al. The operation and efficacy of cryosurgical, nitrous oxide-driven cryoprobe. I. Cryoprobe physical characteristics: their effects on cell cryodestruction. Cryobiology 1994;31:290-304. [PubMed]
- Carpenter RJ 3rd, Neel HB 3rd, Sanderson DR. Cryosurgery of bronchopulmonary structures. An approach to lesions inaccessible to the rigid bronchoscope. Chest 1977;72:279-84. [PubMed]
- Sanderson DR, Neel HB 3rd, Fontana RS. Bronchoscopic cryotherapy. Ann Otol Rhinol Laryngol 1981;90:354-8. [PubMed]
- Rodgers BM, Moazam F, Talbert JL. Endotracheal cryotherapy in the treatment of refractory airway strictures. Ann Thorac Surg 1983;35:52-7. [PubMed]
- Sanderson DR, Neel HB, Payne WS, et al. Cryotherapy for bronchogenic carcinoma: report of a case. Mayo Clin Proc 1975;50:435-7. [PubMed]
- Homasson JP, Renault P, Angebault M, et al. Bronchoscopic cryotherapy for airway strictures caused by tumors. Chest 1986;90:159-64. [PubMed]
- Mathur PN, Wolf KM, Busk MF, et al. Fiberoptic bronchoscopic cryotherapy in the management of tracheobronchial obstruction. Chest 1996;110:718-23. [PubMed]
- Maiwand MO, Zehr KJ, Dyke CM, et al. The role of cryotherapy for airway complications after lung and heart-lung transplantation. Eur J Cardiothorac Surg 1997;12:549-54. [PubMed]
- Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc 2009;6:79-93. [PubMed]
- Rodgers BM, Talbert JL. Clinical application of endotracheal cryotherapy. J Pediatr Surg 1978;13:662-8. [PubMed]
- Majid A, Palkar A, Myers R, et al. Cryotechnology for staged removal of self-expandable metallic airway stents. Ann Thorac Surg 2013;96:336-8. [PubMed]
- Schumann C, Kropf C, Rüdiger S, et al. Removal of an aspirated foreign body with a flexible cryoprobe. Respir Care 2010;55:1097-9. [PubMed]
- Sriratanaviriyakul N, Lam F, Morrissey BM, et al. Safety and Clinical Utility of Flexible Bronchoscopic Cryoextraction in Patients With Non-neoplasm Tracheobronchial Obstruction: A Retrospective Chart Review. J Bronchology Interv Pulmonol 2015;22:288-93. [PubMed]
- Rubio E, Gupta P, Ie S, et al. Cryoextraction: A novel approach to remove aspirated chewing gum. Ann Thorac Med 2013;8:58-9. [PubMed]
- Moorjani N, Beeson JE, Evans JM, et al. Cryosurgery for the treatment of benign tracheo-bronchial lesions. Interact Cardiovasc Thorac Surg 2004;3:547-50. [PubMed]
- Fernando HC, Dekeratry D, Downie G, et al. Feasibility of spray cryotherapy and balloon dilation for non-malignant strictures of the airway. Eur J Cardiothorac Surg 2011;40:1177-80. [PubMed]
- Browning R, Parrish S, Sarkar S, et al. First report of a novel liquid nitrogen adjustable flow spray cryotherapy (SCT) device in the bronchoscopic treatment of disease of the central tracheo-bronchial airways. J Thorac Dis 2013;5:E103-6. [PubMed]
- Walsh DA, Maiwand MO, Nath AR, et al. Bronchoscopic cryotherapy for advanced bronchial carcinoma. Thorax 1990;45:509-13. [PubMed]
- Maiwand MO, Homasson JP. Cryotherapy for tracheobronchial disorders. Clin Chest Med 1995;16:427-43. [PubMed]
- Maiwand MO. The role of cryosurgery in palliation of tracheo-bronchial carcinoma. Eur J Cardiothorac Surg 1999;15:764-8. [PubMed]
- Asimakopoulos G, Beeson J, Evans J, et al. Cryosurgery for malignant endobronchial tumors: analysis of outcome. Chest 2005;127:2007-14. [PubMed]
- Vergnon JM, Mathur PN. Cryotherapy for endobronchial disorders. In: Bolliger CT, Mathur PN, editors. Interventional Bronchoscopy, Vol. 30 of Progress in Respiratory Research. Basel: Karger; 2000;133-45.
- Deygas N, Froudarakis M, Ozenne G, et al. Cryotherapy in early superficial bronchogenic carcinoma. Chest 2001;120:26-31. [PubMed]
- Homasson JP, Pecking A, Roden S, et al. Tumor fixation of bleomycin labeled with 57 cobalt before and after cryotherapy of bronchial carcinoma. Cryobiology 1992;29:543-8. [PubMed]
- Vergnon JM, Schmitt T, Alamartine E, et al. Initial combined cryotherapy and irradiation for unresectable non-small cell lung cancer. Preliminary results. Chest 1992;102:1436-40. [PubMed]
- Forest V, Peoc'h M, Campos L, et al. Effects of cryotherapy or chemotherapy on apoptosis in a non-small-cell lung cancer xenografted into SCID mice. Cryobiology 2005;50:29-37. [PubMed]
- Schumann C, Hetzel J, Babiak AJ, et al. Cryoprobe biopsy increases the diagnostic yield in endobronchial tumor lesions. J Thorac Cardiovasc Surg 2010;140:417-21. [PubMed]
- Hetzel J, Eberhardt R, Herth FJ, et al. Cryobiopsy increases the diagnostic yield of endobronchial biopsy: a multicentre trial. Eur Respir J 2012;39:685-90. [PubMed]
- Babiak A, Hetzel J, Krishna G, et al. Transbronchial cryobiopsy: a new tool for lung biopsies. Respiration 2009;78:203-8. [PubMed]
- Tomassetti S, Wells AU, Costabel U, et al. Bronchoscopic Lung Cryobiopsy Increases Diagnostic Confidence in the Multidisciplinary Diagnosis of Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2015. [Epub ahead of print]. [PubMed]
- Yarmus L, Akulian J, Gilbert C, et al. Cryoprobe transbronchial lung biopsy in patients after lung transplantation: a pilot safety study. Chest 2013;143:621-6. [PubMed]
- Roden AC, Kern RM, Aubry MC, et al. Transbronchial Cryobiopsies in the Evaluation of Lung Allografts: Do the Benefits Outweigh the Risks? Arch Pathol Lab Med 2015. [Epub ahead of print]. [PubMed]
- Fruchter O, Fridel L, Rosengarten D, et al. Transbronchial cryobiopsy in immunocompromised patients with pulmonary infiltrates: a pilot study. Lung 2013;191:619-24. [PubMed]
- Schuhmann M, Bostanci K, Bugalho A, et al. Endobronchial ultrasound-guided cryobiopsies in peripheral pulmonary lesions: a feasibility study. Eur Respir J 2014;43:233-9. [PubMed]
- Poletti V, Casoni GL, Gurioli C, et al. Lung cryobiopsies: a paradigm shift in diagnostic bronchoscopy? Respirology 2014;19:645-54. [PubMed]
- Finley DJ, Dycoco J, Sarkar S, et al. Airway spray cryotherapy: initial outcomes from a multiinstitutional registry. Ann Thorac Surg 2012;94:199-203; discussion 203-4. [PubMed]


