Past, present, and future of endobronchial laser photoresection
Introduction
Management of central airway obstruction is an essential skill for an Interventional Pulmonologist (IP). Thirty percent of lung cancers patients developed central airway involvement during the course of their illness leading to bleeding, post-obstructive pneumonia or respiratory distress (1). Endobronchial therapy of the malignant airway obstruction is considered as a palliative measure or a bridge therapy to the definite treatment of cancer. Several ablative therapies including electrocautery, argon plasma coagulation (APC), cryotherapy and laser photoresection exist in the armamentarium of IP to tackle such presentations. This chapter focuses on the historical perspective, current status, and potentials of lasers in the management of central airway lesions.
History and historical perspective
Therapeutic applications of the Light Amplification by Stimulated Emission of Radiation (LASER) have been recognized since 1960. The CO2 was the first laser that was introduced to the field of medicine. Dr. Patel was the first ever to use CO2 lasers in medicine during early 60s. In 1967, Jako et al., were first to use CO2 laser on a cadaveric larynx. It was also the first laser used in the endobronchial tree. However, CO2 laser has several disadvantages, such as a long wavelength of 10,600 nm requiring a rigid delivery system and suboptimal hemostasis due to its shallow depth of penetration (2). Peter Kiefhaber was first to successfully perform endoscopic argon laser photocoagulation for gastrointestinal bleeding in humans (3). Similar to the CO2 laser however, Argon laser also has shallow depth of penetration and is too weak to produce tissue vaporization. Thus, it has limited ability to stop bleeding from large-bore vessels and its role remains limited to ophthalmology.
Subsequently, Neodymium-Yttrium, Aluminum, Garnet (Nd:YAG) laser was introduced by Geusic et al. at Bell laboratories in 1964. Peter Kiefhaber is considered as a pioneer of using Nd:YAG laser in Medicine. He used Nd:YAG laser photocoagulation to control gastrointestinal bleeding (4). In terms of the laser application in the airways, Toty et al. was first to report treatment of tracheobronchial lesions using Nd:YAG laser at hospital Foch, Lille, France. In 1983, Jean-Francois Dumon, also from France expanded the utility of Nd:YAG laser as the preferred modality for palliation for obstructing malignant lesions of the airways. Its instant popularity was based on the fact that the laser light could be delivered to the distal airways using flexible quarts filaments because of its shorter wave length (1,064 nm) (5). This property of the laser light also made its use possible through a flexible bronchoscope. In subsequent years Nd:YAG laser was also used to treat benign lesions obstructing the large airways (6). Since that time onwards laser photoresection has become a standard treatment in the management of central airway obstruction.
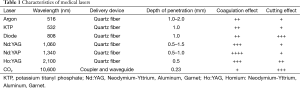
Today there are several different types of laser available for their application in the endobronchial tree (Table 1)
Full table
Technical aspect of lasers
Laser light emission is created by application of a stimulus to the lasing medium. The stimulus that can excite the medium can be any form of energy such as an electrical current or even another laser. The excited electrons of the medium attain a higher level of energy and emit a photon beam as they fall back to the ground energy level. These photons beams are reflected back and forth within the medium by the mirrors placed at the either end, exciting more and more electrons and creating a more powerful light source. Making one of the mirrors partially refractive a fraction of the light is allowed to escape in form of a laser beam.
The laser light thus created has three distinct properties:
- Monochromaticity: all the photons of the laser light have a single wavelength;
- Coherence: laser light waves travel in parallel phase in relation to space and time;
- Collimation: laser light travels in the same direction and with a very narrow beam of divergence allowing persistence of energy over a long distance.
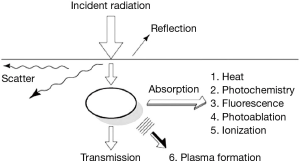
The selection of an appropriate laser for its medical application requires an understanding of laser-tissue interaction. Once the laser light is applied to the tissue it could go either through reflection, absorption, scattering or transmission (Figure 1).
- Reflection is when the radiation is returned back by the tissue surface;
- Absorption is the transfer of the photon energy to the molecules within the tissue that is to be altered. Absorption of the laser light depends on the wavelength of the beam and the color of the tissue. In general darker tissue absorbs more laser light than the paler one. This is especially true for the Nd:YAG laser;
- Scattering occurs when radiation is dispersed in different directions within the tissue without causing desired tissue effect;
- Transmission is the passage of the radiation through the medium without scattering and causing reduced tissue effect. It is governed by the law of inverse absorption; paler the tissue, the greater is the depth of penetration.
The tissue effect of laser also depends on the power and the duration of exposure. This tissue interaction may lead to either thermal, photochemical or electrodynamic effects of the laser beam.
Thermal effect
The thermal effect of the laser beam is based on the principles of molecular agitation. Presently, it is the thermal effect of the Nd:YAG that is most commonly used in interventional pulmonology. The laser medium is neodymium, a pinked-colored rare earth, doped into crystal structure of an yttrium, aluminum and garnet. The wavelength of YAG is 1,064 nm. Interestingly, laser light of this wave length is poorly absorbed by both, the water and the hemoglobin contents of the tissue allowing it penetrate deeply (up to 15 mm), affecting larger area of the tissue creating most appropriate power density for effective coagulation (Figure 2). However, occasionally the depth of penetration is difficult to predict. Hence the laser beam is always fired in the direction of the visible lumen. The Nd:YAG laser wavelength is not visible to human eyes. Thus, a laser light of a visible wavelength is added for the operator to visualize laser beam. Nd:YAG laser is a powerful laser and could produce over 100 Watts. However the devise is relatively bulky, inefficient and expensive.
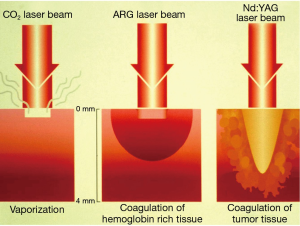
Potassium titanyl phosphate (KTP) laser emits green light at 532 nm. The wavelength which exactly half of the Nd:YAG laser obtained by placing KTP crystal in the path of the laser beam. This double the frequency of the light while halving its wavelength and bringing it in to the visible to the visible green spectrum. In comparison to Nd:YAG, KTP laser light has less depth of penetration and less coagulative property. Thus, it plays an important role in the management of tracheal hemangiomas and tracheoesophageal fistula in pediatric population and in urological procedures (7,8). It is rarely used in adult with large bulky endobronchial lesions where significant debulking is required.
Neodymium: Yttrium-Aluminum-Perovskite (Nd:YAP) laser: in recent years, a more efficient and portable form of thermal laser has been introduced to the medical arena. Nd:YAP laser emits light at 1,340 nm. It has an absorption coefficient in water of 20 times more than Nd:YAG. Theoretically, Nd:YAP has slightly higher wavelength which may provide better coagulation and devascularization than Nd:YAG. In a retrospective review, the use of Nd:YAP laser can effectively restore airway patency without major complications (9). In this study, the most common laser power setting was 20 W/30 Hz.
Homium: Neodymium-Yttrium, Aluminum, Garnet (Ho:YAG) laser: the Ho:YAG operates at the wavelength of 2,100 nm and is absorbed by water approximately 100 times more than Nd:YAG laser. Thus, Ho:YAG laser cuts through the tissue and limits thermal necrosis to the nearby tissue in a similar manner to CO2 laser. Interestingly, Ho:YAG maintains coagulative property similar to Nd:YAG laser. This laser has been used in both malignant and benign endobronchial conditions with minimal postoperative morbidity and mortality (10). Ho:YAG laser fiber can be placed several millimeters from target tissues for rapid vaporization. Alternatively, the laser fiber can be inserted into target tissue. The laser fiber emits photons which are trapped into tissue, providing coagulative effect. In addition to its application in Interventional bronchoscopy, Ho:YAG is widely used for lithotripsy in urologic procedure.
Diode laser has been reported to be used in bronchoscopic procedure. Diode laser has operational wavelength at 808 nm. Tissue absorption of Diode laser is greater than Nd:YAG, the coagulation effect is similar to argon laser, and the cutting effect is comparable to CO2 laser (11).
Indications
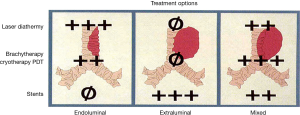
The most common indications of thermal effect of laser photoresection are relieving airway obstruction and alleviating symptoms. Symptoms associated with airway obstructions are shortness of breath, cough, post-obstructive pneumonia and hemoptysis. The mechanism of obstruction is usually classified into benign and malignant. There are three types of airway obstructions: (I) endoluminal growth; (II) extraluminal growth; and (III) mixed type (Figures 3,4). The types of lesion that are most suitable for laser ablation are endobronchial growth with minimal submucosal infiltrations. Table 2 lists the favorable and unfavorable lesions for the laser photoreaction.
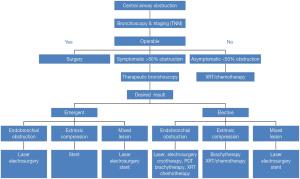
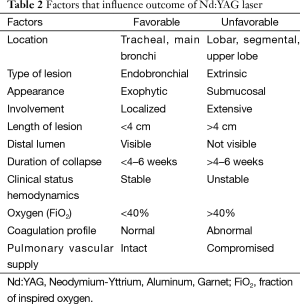
Full table
In terms of malignancy, squamous cell and adenocarcinomas of the lung involving the central airways are the most common indication. The metastatic lesions involving the airways, often treated with laser photocoagulation include thyroid, colon, kidney, esophagus and melanoma. Mechanical debulking without thermal coagulation is at risk of bleeding from neovascularization of the tumor. The depth of penetration of Nd:YAG and Nd:YAP laser provides excellent coagulative effect on blood vessels. The laser causes destruction of tumor cells as well as tissue vaporization.
There are several benign indications for laser therapy. Tracheal stenosis is fairly common complication of prolonged intubation or tracheostomy. Concentric web-like tracheal stricture can be efficiently treated with laser dissection followed by gentle balloon dilatation. Lasers are also used to cut metallic stents or foreign bodies prior to their removal (12).
Contraindications
The laser therapy can be performed safely by a well-trained operator. However, complete or nearly complete airway obstruction without adequate visualization of distal lumen is a relative contraindication due to a possible risk of perforation of the airway. Firing of laser beam with uncertain direction of distal airway can cause injury to major blood vessels or esophagus. In the setting of profound or refractory hypoxemia, unstable cardiovascular status, and bleeding tendency, operator discretion is advisable.
Laser photoresection
The procedure can be carried out either through a flexible or a rigid bronchoscope. The laser fiber is inserted through the working channel of the flexible bronchoscope or the rigid bronchoscope until it protrudes at least 4 mm out of the working channel. The laser delivery system is set on firing mode. The laser fiber tip is kept at least 4 mm away from the lesion and the energy is delivered at the target tissue (Table 3).
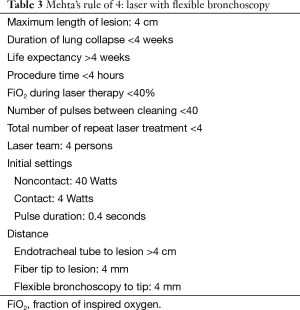
Full table
- Firing angle: the laser beam is fired at the target on an almost parallel angle to the involved airway. The posterior walls of trachea and mainstem bronchi are particularly vulnerable to injury since there is no cartilage to act as a buffer. The laser must not be fired perpendicular to the airway wall to avoid perforation;
- Exposure setting: the laser exposure must be held to what is minimally required. Experimental data have shown that continuous laser exposure at a power setting above 40 Watts creates significant tissue coagulation and may lead to explosion or “popcorn effect”. Therefore, a power setting higher than 40 Watts are usually avoided. The laser energy is delivered in a pulse mode while the continuous mode is avoided;
- Mechanical versus laser resection: for a large exophytic tumor mass obstructing the trachea, a mechanical debulking with the beveled tip of rigid bronchoscope after laser coagulation (low-powered setting; 30 Watts and long duration 1 to 1.5 second) is favored. Meanwhile, a friable tumor can be ablated by laser resection at the higher power setting (40 Watts) and shorter duration (0.5 to 0.7 second).
Complications
Laser therapy is very safe technique in experienced hand, but significant complications can occur from improper assessment of anatomical boundaries, failure to control the airway and inadequate visibility (Table 4). Patient may experience marked fluctuations in oxygen saturation (SpO2) and end-tidal carbon dioxide (ETCO2) in relatively low fraction of inspired oxygen (FiO2) environment maintained to prevent ignition along with use of suction to clear secretions, blood and smoke. Appropriate continuous monitoring is extremely important for the safety of the patients.

Full table
Endobronchial fire is one of the most feared complications of laser therapy. It usually occurs when flammable materials such as endotracheal tube, flexible bronchoscope, and suction catheter get in the path of the laser beam. Fire hazard can be avoided by keeping FiO2 below 40% at all time during laser firing mode. In addition, maintaining power settings to as minimum as possible reduces the risk of the complication.
Massive hemorrhage can occur in case of perforation of a major vessel. This is common with an inability to visualize depth of penetration. High power levels (>40 Watts) or pulse duration (>1 second) are the risk factors for perforation. Nd:YAG laser can also induce damage to the tracheal wall which may lead to tracheal stricture formation. Radial probe endobronchial ultrasound (EBUS) has been used to study the local tracheal anatomy to reduce laser damage however, further experience is warranted (13).
Air embolism has been reported as a complication which is possibly related to the use of air coolant systems at high flows. Minimizing airflow in coolant system and using non-contact probes are recommended to avoid this complication (14).
Dumon has described “Ten Commandments” for safe Nd:YAG laser resection:
- Know the anatomical danger zone (e.g., aortic arch, pulmonary artery, esophagus);
- Have a well-trained laser team (e.g., anesthesia and assistant);
- Proper patient selection;
- Use the rigid bronchoscopic technique;
- Monitor vital signs: oxygen saturation, heart rate, end-tidal CO2;
- Fire the laser beam in parallel to the walls of the airway;
- Avoid laser at high power setting (>40 Watts);
- Do not neglect hemorrhage;
- Terminate each procedure with a through laser irradiation of resected area and a tracheobronchial toilet to remove all secretions and debris;
- Keep the patient under observation in a specially outfitted recovery room for reasonable period of time.
Outcomes
From Cleveland Clinic experience, more than 90% of patients received either excellent or good responses as measured by subjective and objective outcomes. Along with improvement in symptoms, spirometric, performance status, and imaging improvement can be achieved with proper patient selection.
Most of the outcome studies related to laser therapy are neither randomized nor controlled. Comparisons of mortality have been based on anecdotal studies and historical controls. Moreover, most malignant diseases case studies are treated with multiple modalities. Multimodality approach has included both radiation as well as chemotherapy. A study compared outcomes of patients treated with external beam radiation in combination with Nd:YAG laser photoresection with historical control receiving emergent radiation therapy alone and found mortality benefit for the combined mortality (150 vs. 267 days, P=0.04) (15). In addition, inclusion of brachytherapy to Nd:YAG laser photoresection demonstrated improved longevity of the therapeutic effect (mean 16.4+2.5 vs. 40.8+9.4 weeks, P=0.001) with the dual modality (16). In the setting of respiratory failure requiring mechanical ventilation, outcomes including successful extubation and prolonged survival have been reported following the use of Nd:YAG lasers for malignant central airway obstruction (17). In a retrospective comparison of Nd:YAG therapy alone or in conjunction with other modalities, there was improvement in the median time for second intervention by 1.7 months with combined approach (P=0.003 compared to Nd:YAG alone) in non-small cell lung cancer and 3.2 months in all forms of cancer (P=0.002) (18). For a particular malignant cell type (i.e., carcinoid), Nd:YAG laser has been used with curative intent with good success (19).
Photochemical effect
Laser can also cause photochemical reaction in the targeted tissue. Targeted tissue absorbs energy in the form of light which resulted in the creation of transient excited states of molecules. These new chemical species can fall apart, combine with each other, or change to new molecular structure. This photochemical effect has been known as the principle of photodynamic therapy (PDT). In the past this property of Argon laser was used in combination with intravenous photosensitizer Hematoporphyrin-D for the early detection of airway malignancies (20). With an advent of autofluorescence technology and Narrow band imaging this technology has fallen out of researchers’ interest (21).
Electrodynamic effect of laser has little clinical role in pulmonary medicine.
Present status
It needs to be pointed out that in recent years the armamentarium of the IP has expanded with addition of electro-cautery, APC, cryotherapy, microdebrider, brachytherapy as well as stent placement. Most patients are managed in a multimodality fashion for the optimal outcome. In this regard advantages or disadvantages of a specific modality remain difficult to evaluate. This is further compounded by the fact that selection of a specific modality remains operator and institution specific. In absence of well controlled randomized trails even in 2015, experts’ opinion continues to guide the therapeutic approaches.
Future of laser photoresection
We believe that the use of laser photoresection in the management of central airway obstruction is at its peak at present. It has maintained its status with the increasing popularity of the subspecialty of interventional pulmonology. However it is likely that, at least in the developed world, number of patients presenting with large endobronchial lesion will decline in the future. Increasing reliance on the CT scan of the chest and lung cancer screening programs will lead to earlier detection of lung cancer as well as central airway lesions before they become symptomatic. Widespread use of flexible bronchoscopy also will help in early detection of airway lesions before they become critical requiring emergent intervention. It is not too optimistic to accept that reducing popularity of smoking will also reduce in the incidences of lung cancer curtailing the need for laser photoresection (22).
Additionally, recent advancement in personalized treatment for lung cancer using tyrosin kinase inhibitors and monoclonal antibodies is likely to improve the welfare of lung transplant patients by reducing the need for invasive palliative procedures.
Intuitively, as the cost of healthcare continues to escalate future of palliative modalities may heavily depend upon the cost effectiveness of the procedure. In this regard use of reusable tools such as endobronchial electrocautery might be favored over expensive disposable accessories.
While the incidence of lung cancer, benefits of lung cancer screening trials and advances in personalized treatment readjust, the skill of invasive palliative modalities will mainly concentrate at the centers of excellences. But until then, thermal ablative therapy with laser will remain an important tool in the armamentarium of every IP.
Conclusions
Laser photoresection of central airway obstruction is a useful tool in the hands of an experience operator. It is considered as an adjunct to other endobronchial therapeutic modalities. Nd:YAG laser is the most commonly used laser for this indication. In absence of randomized control trials experts’ opining continue to guide its selection and application. “Ten commandments” established by Dr. Dumon are the beacon of light for the IP to attain optimal outcome form the laser photoresection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Minna JD, Higgins GA, Glaistein EJ. Cancer of the lung. In: DeVita VT, Rosenberg SA, Hellman S. Cancer: Principles & Practice of Oncology. Philadelphia: Lippincott Williams & Wilkins, 1989:591-705.
- Jako GJ. Laser surgery of the vocal cords. An experimental study with carbon dioxide lasers on dogs. Laryngoscope 1972;82:2204-16. [PubMed]
- Dwyer RM. The Technique of Gastrointestinal Laser Endoscopy. In: Goldman L, editor. The Biomedical Laser: Technology and Clinical Applications. New York: Springer-Verlag, 1981:255-69.
- Fisher JC. A Brief History of the Nd:YAG Laser. In: Joffe SN, Oguro Y, editors. Advances in Nd:YAG Laser Surgery. New York: Springer-Verlag, 1988:7-9.
- Colt H, Murgu S. Rigid Bronchoscopy with Laser and Stent Placement for Bronchus Intermedius Obstruction from Lung Cancer Involving the Right Main Pulmonary Artery. In: Colt H, Murgu S. Bronchoscopy and Central Airway Disorders: A Patient-Centered Approach. Philadelphia: Elsevier, 2012:269-78.
- Mohan A, Guleria R, Mohan C, et al. Laser bronchoscopy--current status. J Assoc Physicians India 2004;52:915-20. [PubMed]
- Rameau A, Zur KB. KTP laser ablation of extensive tracheal hemangiomas. Int J Pediatr Otorhinolaryngol 2011;75:1200-3. [PubMed]
- Rakoczy G, Brown B, Barman D, et al. KTP laser: an important tool in refractory recurrent tracheo-esophageal fistula in children. Int J Pediatr Otorhinolaryngol 2010;74:326-7. [PubMed]
- Lee HJ, Malhotra R, Grossman C, et al. Initial Report of Neodymium: Yttrium-Aluminum-Perovskite (Nd: YAP) Laser Use During Bronchoscopy. J Bronchology Interv Pulmonol 2011;18:229-32. [PubMed]
- Squiers JJ, Teeter WA, Hoopman JE, et al. Holmium:YAG laser bronchoscopy ablation of benign and malignant airway obstructions: an 8-year experience. Lasers Med Sci 2014;29:1437-43. [PubMed]
- Dalar L, Karasulu L, Sökücü SN, et al. Diode laser in the endoscopic treatment of obstructive airway disease: two years experience in Istanbul. Thorac Cardiovasc Surg 2012;60:140-4. [PubMed]
- Mehta AC, Harris RJ, De Boer GE. Endoscopic management of benign airway stenosis. Clin Chest Med 1995;16:401-13. [PubMed]
- Murgu SD, Colt HG, Mukai D, et al. Multimodal imaging guidance for laser ablation in tracheal stenosis. Laryngoscope 2010;120:1840-6. [PubMed]
- Golish JA, Pena CM, Mehta AC. Massive air embolism complicating Nd-YAG laser endobronchial photoresection. Lasers Surg Med 1992;12:338-42. [PubMed]
- Desai SJ, Mehta AC, VanderBrug Medendorp S, et al. Survival experience following Nd:YAG laser photoresection for primary bronchogenic carcinoma. Chest 1988;94:939-44. [PubMed]
- Shea JM, Allen RP, Tharratt RS, et al. Survival of patients undergoing Nd:YAG laser therapy compared with Nd:YAG laser therapy and brachytherapy for malignant airway disease. Chest 1993;103:1028-31. [PubMed]
- Colt HG, Harrell JH. Therapeutic rigid bronchoscopy allows level of care changes in patients with acute respiratory failure from central airways obstruction. Chest 1997;112:202-6. [PubMed]
- Han CC, Prasetyo D, Wright GM. Endobronchial palliation using Nd:YAG laser is associated with improved survival when combined with multimodal adjuvant treatments. J Thorac Oncol 2007;2:59-64. [PubMed]
- Fuks L, Fruchter O, Amital A, et al. Long-term follow-up of flexible bronchoscopic treatment for bronchial carcinoids with curative intent. Diagn Ther Endosc 2009;2009:782961.
- Hirsch FR, Franklin WA, Gazdar AF, et al. Early detection of lung cancer: clinical perspectives of recent advances in biology and radiology. Clin Cancer Res 2001;7:5-22. [PubMed]
- Leong S, Shaipanich T, Lam S, et al. Diagnostic bronchoscopy--current and future perspectives. J Thorac Dis 2013;5 Suppl 5:S498-510. [PubMed]
- Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on lung cancer risk. JAMA 2005;294:1505-10. [PubMed]