
The pathological oral cavity as a preventable source of postoperative pneumonia in thoracic surgery: a prospective observational study
Introduction
Perioperative management in thoracic surgery is highly standardised, and with minimally invasive resections, the rate of postoperative complications in routine operations continually decreases. However, thoracic surgeons are confronted with an increasingly older collective of patients with multiple comorbidities. In the last decades, several developments like minimally-invasive approaches and enhanced recovery after surgery (ERAS) contributed to the safety profile and are of particular benefit in older patients (1). Despite all improvements, serious postoperative complications still occur (2,3). One of the most frequent severe postoperative complications is postoperative pneumonia. Although there were several attempts to minimize the risk for postoperative pneumonia, little is known about the exact origin or whether there are avoidable factors. One possible source for bacteria entry may be the oral cavity. During the staging period (e.g., via bronchoscopy), and later during intubation, bacteria may spread from the dental biofilm into the lungs. We hypothesized that the oral cavity is a source for postoperative pneumonia. Therefore, we designed a prospective observational study to analyze the link between the health status of the oral cavity and postoperative outcomes, especially postoperative pneumonia. We present the following article in accordance with the STROBE reporting checklist (4) (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1178/rc).
Methods
We conducted a prospective observational study, which was approved by the Ethics Committee at the Medical Faculty of the University Essen (17-7822-BO). The study is registered in the German clinical trial registry (DRKS00017762), which is a WHO-recognized primary registry for Germany. All patients signed written informed consent to be eligible for study participation. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study design
The study was designed as an investigator-initiated, prospective, single-center study to analyze the link between the occurrence of postoperative respiratory complications and the health status of the oral cavity.
Routine management
All patients underwent thoracic surgery in our department between 10/02/18 and 04/29/20. Inclusion and exclusion criteria are shown in Table 1. Data on demographics, preexisting conditions, and postoperative complications were recorded prospectively. We measured preoperative height and weight, which were used to calculate body mass index (BMI). All patients received a twelve-channel electrocardiogram, blood gas analysis, and blood analysis at a minimum. In cases of planned lung resection, patients also underwent functional evaluation according to the current guidelines of the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) (5). In cases of proven or suspected lung cancer, patients were staged according to the current guidelines of ESTS and the European Society for Medical Oncology (ESMO). In these cases, patients received Fluoro-2-deoxy-D-glucose (FDG)-positron emission tomography (PET), computed tomography, brain imaging [magnetic resonance imaging (MRI) or contrast-enhanced computed tomography (CE-CT)], and if necessary, endobronchial ultrasound transbronchial needle aspirations (EBUS-TBNA) for invasive mediastinal staging. Patients were administered 1g intravenous ampicillin at the beginning of the operation. In case of lung resection, the administration was extended to the third postoperative day. 4 g/0.5 g Piperacillin/Tazobactam was given to patients after neoadjuvant treatment. Postoperative complications and 30-day mortality were prospectively recorded.
Table 1
| Inclusion | Exclusion |
|---|---|
| Elective thoracic surgical intervention | Older than 99 years |
| Informed consent | Pneumonia |
| Older than 18 years | Acute systemic infection |
| Immunosuppression |
Examination of oral cavity/dental examination
All patients who gave informed consent after screening eligibility for participation in the study were examined by an experienced dentist one day prior to surgery. The dentist was blinded to the pulmonary function testing. We evaluated the following conditions: dental caries, periodontal disease, missing teeth, teeth treated by a dentist, and undiagnosed swallowing disorders leading to an increased risk of aspiration. The health status of the oral cavity was assessed visually by using a mirror. We focused on possible sources causing postoperative pneumonia or triggering other inflammatory based events. Therefore, we were interested in the current and past caries burden. A subgroup of patients with a high caries burden (more than ten teeth with active caries) was identified based on the adapted risk model of Terpenning et al. (6). We recorded active caries and the number of affected teeth. We also investigated whether periodontal disease was present in the oral cavity. Active periodontal disease, lost teeth and clinical signs of acute infection were also recorded. We also investigated if patients had regular dental visits and if there were (un-)treated teeth. Finally, we also investigated the swallowing function/undiscovered signs of Dysphagia and/or tendency for aspiration by having patients take a sip of water.
Definition of common perioperative complications and mortality
We defined perioperative mortality as death for any reason in a period of 30 days starting from the first day after operation. A postoperative complication was defined as any deviation from the normal postoperative course (7). Prolonged air leak was defined according to the definition of the ESTS as an air leak lasting longer than five days after surgery (8). Pneumonia was defined by the definition of Centers for Disease Control and Prevention criteria for nosocomial pneumonia (9). Pleural empyema was defined by purulent pleural effusion with or without detection of bacteria. For respiratory failure the definition of the ERS was used (10). The classification of Clavien-Dindo was used to grade complications (7).
Statistical analysis
Statistical analysis was conducted by using MedCalc software Version 11.6.1.0 (MedCalc software, Broekstraat 52, 9030 Mariakerke, Belgium). Statistical advice was provided by the Institute of Medical Informatics, Biometry and Epidemiology, University Medicine Essen.
The parameters of oral health and surgical outcome were analyzed by crosstabs and the Fischer’s exact test. The association between patient characteristics and surgical outcome was analyzed by logistic regression. The difference between different groups or clinical parameters was analyzed by the T-test or Mann-Whitney test. Confounding factors were tested by the Cochran-Mantel-Haenszel test. The Breslow-Day-Tarone test was performed to test homogeneity of the odds ratios (OR). A P value <0.05 was considered significant. Figures were given as bars or dots.
Results
Characteristics of the study cohort
We included 230 patients [128 male (56%) and 102 female (44%)] in our study. All patient characteristics are shown in Table 2. The median age was 64 years (range 16 to 89 years). The study population consisted of 53% (N=122) ex-smokers (>6 months of nicotine abstinence), 33% (N=77) active smokers, and 14% (N=31) never-smokers. The median BMI was 25 kg/m2 (range, 17 to 55 kg/m2). The median preoperative serum CRP level was 0.7 mg/dl (range, 0 to 29 mg/dL). The median preoperative creatinine serum level was 0.8 mg/dL (range, 0.4 to 2.4 mg/dL), and the median preoperative white blood cell count (WBC) was 7,600 cells/µL (range, 800 to 20,000 cells/µL). The median preoperative serum albumin level was 4.3 g/dL (range, 2.5 to 5.4 g/dL). Preoperative lung functional assessments revealed a predicted median forced expiratory volume in one second (FEV1) of 71% (range, 18% to 123%).
Table 2
| Variables | Cohort studied (N=230) |
|---|---|
| Female/male | 102 (44%)/128 (56%) |
| Median age (years) | 64 (range, 16–89) |
| Median FEV1 (range) | 71% predicted (range, 18% to 123%) |
| Median BMI (kg/m2) | 25 (range, 17 to 55) |
| Median WBC (cells/µL) | 7,600 (range, 800 to 20,000) |
| Median CRP (mg/dL) | 0.7 (range, 0 to 29) |
| Median albumin (g/dL) | 4.3 (range, 2.5 to 5.4) |
| Median creatinine (mg/dL) | 0.8 (range, 0.4 to 2.4) |
| Smoking status | 53% (N=122) ex-smokera |
| 33% (N=77) smoker | |
| 14% (N=31) never smoker |
a, no nicotine abuse for at least six months; FEV1, forced expiratory volume in one second; BMI, body mass index; WBC, white blood cells; CRP, C reactive protein.
Characteristics of surgical outcomes
The indication for curative intent surgery was lung cancer in 122 patients. Other indications for surgery were listed in Table 3.
Table 3
| Variables | N (total N=230) |
|---|---|
| Indication for surgery | |
| NSCLC | 122 |
| Metastasis | 33 |
| Pleural effusion | 25 |
| Benign nodule | 20 |
| Pneumothorax/Bulla | 5 |
| Tracheal pathology | 4 |
| Mediastinal lymphadenopathy | 6 |
| Mesothelioma | 3 |
| Adenoidcystic carcinoma | 1 |
| Port-catheter implantation for chemotherapy | 4 |
| Chest wall tumour | 2 |
| Chronic empyema | 5 |
| Type of surgical intervention | |
| Lobectomy | 93 |
| Extended lobectomy (with chest wall) | 7 |
| Wedge resection | 27 |
| Metastasectomy | 22 |
| Bilobectomy | 4 |
| Pneumonectomy | 5 |
| Sleeve lobectomy | 8 |
| Airway sleeve | 1 |
| Tracheal resection | 4 |
| Operation of the chest wall | 5 |
| Pneumothorax surgery/Bulla resection | 5 |
| Port-catheter implantation | 4 |
| LVRS | 1 |
| EPP or eP/D | 3 |
| Empyemectomy | 5 |
| Right/left side/both* | 115/89/16 |
| Approach | |
| Thoracotomy | 119 |
| RATS/VATS | 101 |
| Other | 10 |
*, mediastinoscopy and cervical tracheal resection was not assigned to a side (N=10). NSCLC, non-small cell lung cancer; LVRS, lung volume reduction surgery; EPP, extrapleural pleuropneumonectomy; eP/D, extended pleurectomy/decortication; RATS, robotic-assisted thoracic surgery; VATS, video-assisted thoracic surgery.
We performed in 53% (N=123) patients a thoracotomy and in 47% (N=107) a minimal invasive approach. In total we performed 93 lobectomies, seven extended lobectomies (including resection of chest wall or vena cava), four bilobectomies five pneumonectomies, eight sleeve lobectomies and one lung sparing sleeve of the main bronchus. We also performed 49 atypical resections, four tracheal resections, five operations of the chest wall and in five times an operation for pneumothorax/Bulla. In 29 patients, we performed a minor surgery. In one case, we performed a lung volume reduction surgery (LVRS). In eight cases, we performed surgery for mesothelioma or other pleural disease.
There were no intraoperative complications and the 30-day mortality rate was 0% (Table 4). The rate of postoperative complications was 17% (N=35). Postoperative pneumonia occurred in 4.3% (N=10) of cases. In two cases of pneumonia, sampling of tracheal aspirates was done and in both, Pseudomonas aeruginosa was detected. The most common complication was prolonged air leak (>5th day after operation) with 6.1% (N=14). Other rare complications included the following: pleural effusion and reinsertion of a chest drain 2.2% (N=5), hematothorax 1.3% (N=3), postoperative empyema 1.3% (N=3), and chylothorax, renal failure, and wound infection, each 0.4% (N=1).
Table 4
| Variables | N (total N=230) |
|---|---|
| Intraoperative complications | None |
| Postoperative complications | |
| Prolonged air leak | 14 |
| Postoperative pneumonia | 10 |
| Collection of pleural effusion* | 5 |
| Hematothorax | 3 |
| Chylothorax | 1 |
| Wound infection | 1 |
| Renal failure | 1 |
| 30-day mortality | 0 |
| Classification of Clavien-Dindo | |
| I | 15 |
| II | 11 |
| IIIa | 5 |
| IIIb | 3 |
| IVa | 1 |
*, with reinsertion of a chest drain after removal of the intraoperative chest drain.
Health status of oral cavities
Only 38.3% (N=88) of patients attended regular dental visits and had clinical evidence of frequently treated teeth (Table 5). The majority of patients, 90% (N=208), had lost a minimum of one tooth, and only 10% (N=22) of patients had a complete set of teeth. A high proportion of patients [61.7% (N=142)] had active caries, and only 38.3% (N=88) of patients were free of caries. Only 17.4% (N=40) of patients had no sign of periodontal disease. Progressive/aggressive periodontal disease were detected in 42.6% (N=98) of patients, and 40% (N=92) of patients had chronic periodontal disease. In two patients, an undiagnosed tendency for aspiration with signs of dysphagia was noticed.
Table 5
| Variables | N (total N=230) |
|---|---|
| Regular visits to the dentist/clinical evidence of frequently treated teeth | |
| Yes | 88 (38.3%) |
| No | 142 (61.7%) |
| Tooth lost | |
| Yes | 208 (90%) |
| No | 22 (10%) |
| Caries | |
| Yes | 142 (61.7%) |
| No | 88 (38.3%) |
| Peridontal disease | |
| Yes | 40 (17.4%) |
| No | 192 (82.6%) |
Patients with frequent dental visits and treated teeth had a lower risk for postoperative complications compared with patients without regular visits (OR 0.3, P<0.02) (Figure 1A). Patients with active caries had a considerably elevated risk for postoperative complications (OR 2.5, P<0.03) (Figure 1B). Periodontal disease did not significantly elevate the risk for postoperative complications (OR 2.1, P=0.2) (Table 6, Figure 1C).
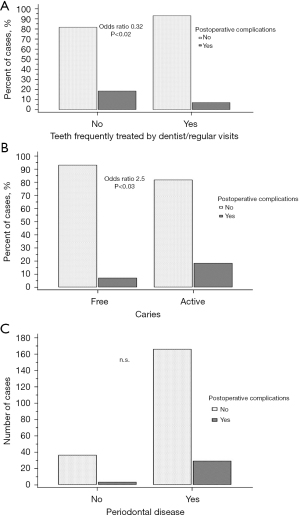
Table 6
| Variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Frequently dental visits | 0.3 | 0.1 to 0.8 | <0.02 |
| Caries | 2.5 | 1.1 to 6.2 | <0.03 |
| Periodontal disease | 2.1 | 0.6 to 7.2 | 0.2 |
CI, confidence interval.
We also investigated if the risk for postoperative pneumonia was elevated for patients who regularly visited their dentist and found a strong but statistically insignificant association (OR 5.8, P=0.09). In addition, we also found no significant association between patients with active caries and postoperative pneumonia (OR 2.5, P=0.2) (Figure 2A).
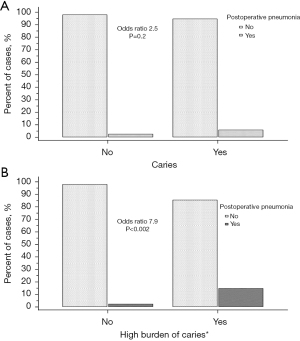
We further analyzed the association of postoperative pneumonia in a subgroup of patients with a very high burden of caries (>ten teeth with active caries) and found a high level of association (OR 7.9, P<0.002) (Figure 2B).
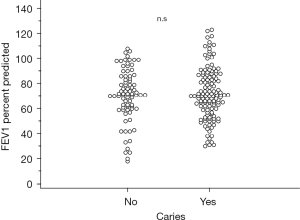
Additionally, a logistic regression analysis was performed to evaluate whether the percent predicted FEV1, albumin, creatinine, CRP, WBC, or BMI were associated with postoperative complications (Table 7). Only the predicted FEV1 percent was significantly associated with postoperative complications (OR 0.9, P<0.02). Median percent predicted FEV1 values did not significantly differ in patients with active caries compared to those without (71%, range 18% to 108% vs. 70%, range 30% to 123%; P=0.4) and was not a confounding factor for caries in the Cochran-Mantel-Haenszel test for postoperative complications or postoperative pneumonia (Figure 3).
Table 7
| Variables | Odds ratio | 95% CI | Std. Error | P value |
|---|---|---|---|---|
| FEV1% | 0.9 | 0.94 to 0.99 | 0.01 | <0.02 |
| BMI | 0.9 | 0.8 to 1 | 0.04 | 0.7 |
| Albumin | 1 | 0.2 to 3 | 0.6 | 0.9 |
| WBC | 1 | 0.9 to 1.2 | 0.07 | 0.2 |
| CRP | 0.8 | 0.7 to 1 | 0.09 | 0.1 |
| Creatinine | 0.4 | 0.05 to 3.5 | 1 | 0.4 |
FEV1, forced expiratory volume in one second; BMI, body mass index; WBC, white blood cells; CRP, C reactive protein; CI, confidence interval; Std., standard.
Discussion
Despite all the advances that have been made over the last decades in the field of thoracic surgery, pneumonia, respiratory insufficiency, and other postoperative complications associated with inflammatory reactions are still a relevant problem in daily practice. Several attempts have been made in the past to identify risk factors for postoperative complications. Pulmonary function testing (and in particular, percent predicted FEV1) is still one of the best evaluated and strongest predictors for the occurrence of postoperative complications (11-13).
However, functional lung testing alone is not sufficient to identify all patients who are at increased risk of developing postoperative complications, and a broad range of publications deal with other predicting factors or risk models (14,15). However, a clear limitation is that the majority of these factors may identify high risk patients but cannot be modified preoperatively (2). A heavy smoker with a lowered FEV1 requiring lung surgery for non-small cell lung cancer (NSCLC) may be preoperatively identified as a high-risk candidate but still needs treatment, and the decision is frequently limited to performing surgery or choosing alternative options. Therefore, we aimed to identify factors that could be identified preoperatively and could also be modified. We hypothesized that the biofilm from the teeth and the oral cavity transfers bacteria via the trachea in the lungs and contributes to postoperative complications like pneumonia. With an increase of caries and poor dental hygiene, more bacteria are carried over, and the risk for postoperative complications increases (16-18). There is some evidence for this hypothesis: The direct anatomical connection between the oral cavity and the respiratory tract results in a topological continuity of the microbiome (19-21). Therefore, it is not uncommon to culture anaerobes originating from the periodontal pocket from pneumonia aspirates (22,23). Mehtonen et al. also demonstrated in a study with 1,592 young Finnish adults that pathological conditions of the oral cavity (like caries) in young and healthy populations are associated with a higher risk for lower respiratory tract infections (24).
In addition, there is a strong and significant link between pathological oral health and many other pulmonary diseases (25). Chronic obstructive pulmonary disease (COPD), for example, is a widespread disease and common among patients undergoing thoracic surgery. There is robust evidence that COPD is linked to caries and periodontal disease (26). Both impact lung function and frequency of exacerbation (27,28).
In mechanically ventilated patients, protective reflexes like coughing and swallowing are suspended. Consecutive silent aspiration with oral pathogens results and may be a source of infection in the postoperative setting. In a study with 341 patients who had undergone an abdominal surgical procedure, Nishikawa et al. demonstrated that periodontal disease was a risk factor for infectious postoperative complications (29). The presence of periodontal disease was also associated with postoperative pneumonia in a study with patients who underwent brain surgery (30). Patients with esophageal cancer have a remarkably high risk for postoperative pneumonia, and therefore, prevention is highly relevant in the surgical management of these patients. In a prospective study from Japan, patients who underwent esophagectomy were investigated, and a positive preoperative dental plaque culture was highly predictive for the occurrence of postoperative pneumonia. Additionally there was a large overlap of pathogenic bacteria between the preoperatively sampled oral culture and the culture from the postoperative sputum sample in patients who suffered from pneumonia (31). In another large study with over 500 patients who underwent esophagectomy, Sato et al. demonstrated that preoperative dental examination and dental care dramatically reduced the risk for occurrence of postoperative pneumonia by 50 percent (32). Interestingly, even the rate of anastomotic leakage decreased, although it was not significant. There were several interventional studies trying to influence the rate of postoperative pneumonia by different preoperative interventions such as simple tooth brushing, professional dental care, or oral rinses with chlorhexidine (33-36). In all studies, a significant decrease in the incidence of postoperative pneumonia could be observed.
In this study, we aimed to identify the influence of pathological conditions in the oral cavity on the occurrence of postoperative pneumonia (and other inflammatory triggered complications) in patients who underwent thoracic surgery. This is the first study investigating the oral health care status in a large cohort undergoing thoracic surgery and correlating it with postoperative complications. In summary, the majority of patients had a poor oral health status. Only 39% attended regular visits to the dentist, and caries and/or periodontal disease was often detected. Surprisingly, two patients had an undiagnosed tendency for aspiration that otherwise would not have been discovered. Patients who regularly visit the dentist had a significantly lower rate of complications. Caries was also significantly associated with a higher rate of complications, whereas the median percent predicted FEV1 was equally distributed in patients with and without caries (Figure 3). There was a strong but insignificant association between caries and postoperative pneumonia, which was most likely insignificant due to the group size. However, in a study with an eight-year observation period, in a large collective with more than 350 subjects, Terpenning et al. investigated risk factors for aspiration pneumonia and found that every decayed tooth, which is identical to dental caries, was associated with a higher risk for pneumonia (OR 1.2) (6). Therefore, we also investigated the group of patients with a high burden of caries (> ten decayed teeth) and found a high risk for postoperative pneumonia (OR 7.9, P<0.002).
We also performed a logistic regression, and neither BMI and albumin as indicators of nutritional status nor WBC, CRP, or creatinine were statistically significant for an elevated risk for postoperative complications. The only factor that was predictive for postoperative complications was percent predicted FEV1. However, this factor is, in most cases, hardly modifiable.
Limitations of the study
In this proof-of-concept study, we were able to demonstrate that the health status of the oral cavity has an influence on the postoperative outcome after thoracic surgery. This study is limited to a single European center with mainly Caucasian patients. Additionally, other factors and cofounders influencing the outcome cannot be ruled out. In the most interesting group of patients with anatomical resections, we could only detect a strong trend without significance due to the group size. Further investigations would be necessary to uncover an indirect influence.
Whether preoperative interventions impact the complications rates remains to be determined in a prospective, randomized trial.
Conclusions
Pathological conditions like caries and periodontal disease seem to have an influence on the rate of postoperative complications after thoracic surgery, especially pneumonia. In contrast to FEV1, pathological conditions in the oral cavity are addressable. The reduction of the biofilm and of the load of bacterial contamination are feasible ways to reduce the rate of complications. An assessment of the oral microbiome and its association with respiratory infections after surgery are warranted.
Acknowledgments
Funding: This work was supported by the Open Access Publication Fund of the University of Duisburg-Essen.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1178/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1178/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1178/coif). KD reports receiving different fees for research/lectures/consulting, but all issues are not related to this study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the University Essen (17-7822-BO). All patients signed written informed consent to be eligible for study participation.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zaatar M, Stork T, Valdivia D, et al. Minimal-invasive approach reduces cardiopulmonary complications in elderly after lung cancer surgery. J Thorac Dis 2020;12:2372-9. [Crossref] [PubMed]
- Agostini P, Cieslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 2010;65:815-8. [Crossref] [PubMed]
- Sandri A, Papagiannopoulos K, Milton R, et al. Major morbidity after video-assisted thoracic surgery lung resections: a comparison between the European Society of Thoracic Surgeons definition and the Thoracic Morbidity and Mortality system. J Thorac Dis 2015;7:1174-80. [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. [Crossref] [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- Terpenning MS, Taylor GW, Lopatin DE, et al. Aspiration pneumonia: dental and oral risk factors in an older veteran population. J Am Geriatr Soc 2001;49:557-63. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Ploenes T, Kyritsis I, Mardanzai K, et al. A Prospective Study Investigating Blood Patch Pleurodesis for Postoperative Air Leaks After Pulmonary Resection. J Surg Res 2020;255:240-6. [Crossref] [PubMed]
- Rotstein C, Evans G, Born A, et al. Clinical practice guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Can J Infect Dis Med Microbiol 2008;19:19-53. [Crossref] [PubMed]
- Roussos C, Koutsoukou A. Respiratory failure. Eur Respir J Suppl 2003;47:3s-14s. [Crossref] [PubMed]
- Pierce RJ, Copland JM, Sharpe K, et al. Preoperative risk evaluation for lung cancer resection: predicted postoperative product as a predictor of surgical mortality. Am J Respir Crit Care Med 1994;150:947-55. [Crossref] [PubMed]
- Brunelli A, Refai M, Salati M, et al. Predicted versus observed FEV1 and DLCO after major lung resection: a prospective evaluation at different postoperative periods. Ann Thorac Surg 2007;83:1134-9. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Brunelli A, Salati M, Rocco G, et al. European risk models for morbidity (EuroLung1) and mortality (EuroLung2) to predict outcome following anatomic lung resections: an analysis from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg 2017;51:490-7. [PubMed]
- Brunelli A, Morgan-Hughes NJ, Refai M, et al. Risk-adjusted morbidity and mortality models to compare the performance of two units after major lung resections. J Thorac Cardiovasc Surg 2007;133:88-96. [Crossref] [PubMed]
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007;369:51-9. [Crossref] [PubMed]
- Sands KM, Wilson MJ, Lewis MAO, et al. Respiratory pathogen colonization of dental plaque, the lower airways, and endotracheal tube biofilms during mechanical ventilation. J Crit Care 2017;37:30-7. [Crossref] [PubMed]
- Tada A, Miura H. Prevention of aspiration pneumonia (AP) with oral care. Arch Gerontol Geriatr 2012;55:16-21. [Crossref] [PubMed]
- Charlson ES, Bittinger K, Haas AR, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med 2011;184:957-63. [Crossref] [PubMed]
- Bassis CM, Erb-Downward JR, Dickson RP, et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 2015;6:e00037. [Crossref] [PubMed]
- Morris A, Beck JM, Schloss PD, et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med 2013;187:1067-75. [Crossref] [PubMed]
- Wu BG, Segal LN. The Lung Microbiome and Its Role in Pneumonia. Clin Chest Med 2018;39:677-89. [Crossref] [PubMed]
- Persson GR, Hitti J, Paul K, et al. Tannerella forsythia and Pseudomonas aeruginosa in subgingival bacterial samples from parous women. J Periodontol 2008;79:508-16. [Crossref] [PubMed]
- Mehtonen IT, Rantala AK, Hugg TT, et al. Dental caries is associated with lower respiratory tract infections: A population-based cohort study. Respir Med 2019;158:1-5. [Crossref] [PubMed]
- Manger D, Walshaw M, Fitzgerald R, et al. Evidence summary: the relationship between oral health and pulmonary disease. Br Dent J 2017;222:527-33. [Crossref] [PubMed]
- Lopez-de-Andrés A, Vazquez-Vazquez L, Martinez-Huedo MA, et al. Is COPD associated with periodontal disease? A population-based study in Spain. Int J Chron Obstruct Pulmon Dis 2018;13:3435-45. [Crossref] [PubMed]
- Zhou X, Han J, Liu Z, et al. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2-year pilot randomized controlled trial. J Clin Periodontol 2014;41:564-72. [Crossref] [PubMed]
- Bergström J, Cederlund K, Dahlén B, et al. Dental health in smokers with and without COPD. PLoS One 2013;8:e59492. [Crossref] [PubMed]
- Nishikawa M, Honda M, Kimura R, et al. Clinical impact of periodontal disease on postoperative complications in gastrointestinal cancer patients. Int J Clin Oncol 2019;24:1558-64. [Crossref] [PubMed]
- Bágyi K, Haczku A, Márton I, et al. Role of pathogenic oral flora in postoperative pneumonia following brain surgery. BMC Infect Dis 2009;9:104. [Crossref] [PubMed]
- Akutsu Y, Matsubara H, Okazumi S, et al. Impact of preoperative dental plaque culture for predicting postoperative pneumonia in esophageal cancer patients. Dig Surg 2008;25:93-7. [Crossref] [PubMed]
- Sato Y, Motoyama S, Takano H, et al. Esophageal Cancer Patients Have a High Incidence of Severe Periodontitis and Preoperative Dental Care Reduces the Likelihood of Severe Pneumonia after Esophagectomy. Dig Surg 2016;33:495-502. [Crossref] [PubMed]
- Yamada Y, Yurikusa T, Furukawa K, et al. The Effect of Improving Oral Hygiene through Professional Oral Care to Reduce the Incidence of Pneumonia Post-esophagectomy in Esophageal Cancer. Keio J Med 2019;68:17-25. [Crossref] [PubMed]
- Akutsu Y, Matsubara H, Shuto K, et al. Pre-operative dental brushing can reduce the risk of postoperative pneumonia in esophageal cancer patients. Surgery 2010;147:497-502. [Crossref] [PubMed]
- Shin JH, Kunisawa S, Fushimi K, et al. Effects of preoperative oral management by dentists on postoperative outcomes following esophagectomy: Multilevel propensity score matching and weighting analyses using the Japanese inpatient database. Medicine (Baltimore) 2019;98:e15376. [Crossref] [PubMed]
- Nicolosi LN, del Carmen Rubio M, Martinez CD, et al. Effect of oral hygiene and 0.12% chlorhexidine gluconate oral rinse in preventing ventilator-associated pneumonia after cardiovascular surgery. Respir Care 2014;59:504-9. [Crossref] [PubMed]


