Biopsy testing in an inoperable, non-small cell lung cancer population—a retrospective, real-life study in Sweden
Introduction
Lung cancer is a leading cause of cancer-related death worldwide (1). Overall 5-year survival rates vary from 15–31%, with an exponential decrease for individuals with late-stage disease, in which approximately 70% of patients with non-small cell lung cancer (NSCLC) are diagnosed (1-3). In Sweden, the overall incidence is approximately 40 cases per 100,000 inhabitants and the 5-year survival has been reported to be approximately 12% for men and 15% for women (4,5).
Although most lung cancer patients present with advanced disease and are diagnosed based mainly on symptoms (3,6,7), new treatment strategies require a change in lung cancer diagnosis. Traditionally, histological typing and stage at diagnosis has driven treatment strategies (3,7), but the discovery of new tumour biomarkers and molecular targets for customized therapies has raised the importance of molecular typing to individualize treatments with the aim of improving treatment outcomes (3,8-11).
Overall, lung cancer has a poor prognosis and rapid treatment initiation is vital, not least considering the patients’ psychological well-being. As new biomarkers become available and lung cancer treatment will depend not only on traditional chemotherapy, robust and efficient techniques to obtain sufficient lung cancer tumour samples for accurate and fast molecular diagnosis to guide treatment decisions are essential (3,7,9). To potentiate this, increased awareness among healthcare professionals on the importance of availability of qualitative malignant tissue for diagnosis and treatment of lung cancer patients is needed (3,9).
A number of genetic abnormalities (mutations, translocations, etc.) in genes coding for key cellular proteins are associated with lung cancer, for example, the epidermal growth factor receptor (EGFR) gene, the anaplastic lymphoma kinase (ALK), and the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) (3,8-10). The EGFR is a transmembrane tyrosine kinase receptor, which, when mutated and/or over-expressed, leads to uncontrolled cell proliferation via a number of signalling pathways (8,11). It can be specifically targeted by specific tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib (9,11-14). To test for EGFR mutation status, tissue obtained by core needle biopsy with a high ratio of malignant cells to ‘contaminating’ benign/non-lesional cells is preferable (3,9).
The diagnosis and staging by histological typing and molecular status of a tumour is required for optimal choice of treatment in lung cancer. This retrospective study was done to determine how lung cancer patients are currently diagnosed in Sweden, based on real-life use of different diagnostic techniques, for example, computed tomography (CT)-guided biopsy, and to investigate any complications that may preclude their use and time to diagnosis.
Methods
Study design, protocol and data sources
This was a retrospective, observational study of patient medical records conducted in Sweden to investigate the status of lung cancer biopsy testing between 1 June 2009 and 31 May 2010. Participating sites included the 30–50 most recently diagnosed, inoperable lung cancer patients, starting from the latter date and consecutively and going back in time. Data were entered into an electronic, web-based data capture system. The social security numbers of identified patients were replaced with study identification numbers prior to data processing.
The study protocol was reviewed and approved by the regional ethics committee in Uppsala, Sweden (Reference number 2010/173) and registered at ClinicalTrials.gov (clinical trial identifier NCT 01139619). The study was conducted in accordance with the principles stated in the Declaration of Helsinki, ICH GCPs and the applicable legislations on Non-Interventional Studies.
Patient population and data collection
Male and female patients with a recorded diagnosis of inoperable malignant tumour of bronchus and lung could be included [diagnosis code C34 according to International Classification of Diseases, 10th revision, Clinical Modification (ICD-10-CM)]. Patients were retrospectively identified using medical records at three geographically widespread pulmonology clinics (Gävle, Linköping and Luleå). Data (e.g., demography, smoking history, emergency and/or pulmonology clinic contact history, lung function, haematological status, co-morbidities, antithrombotic treatment at diagnosis [Anatomical Therapeutic Classification System code, ATC B01A], cytology, histology and EGFR testing) were collected consecutively from 31st May 2010 going back in time for up to one year or until 30–50 patients who fulfilled the inclusion criteria were recruited. Patients with diagnosis codes ICD-10-CM C34.9b (small-cell lung cancer) and/or C34.9h (carcinoid) were excluded.
Data on performed diagnostic sampling methodology were collected for each patient, including date of bronchoscopy, biopsy (performed by a pulmonologist) and CT-guided biopsy, tumour sampling technique and any associated complications.
Data on first-line treatment and disease progression one year following diagnosis were recorded.
Study measures and statistical methods
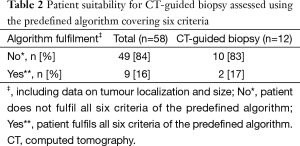
Criteria for patient suitability for CT-guided biopsy were examined using a predefined algorithm, setting theoretical limits for the possibility to perform the sampling based on six measures: (I) localisation of tumour ≤10 cm from the thoracic wall; (II) size of tumour ≥1 cm; (III) forced expiratory volume in one second (FEV1) ≥1 L; (IV) blood saturation ≥90%; (V) normal P-PK (prothrombin time) and P-APTT (activated partial prothromboplastin time); and (VI) tumour ≥1 cm from great vessels. Theoretically, patients should have fulfilled all six criteria to be suitable for a CT-guided, core needle biopsy.
The main statistical analyses were descriptive and data are presented using standard summary measures, such as mean [standard deviation (SD)] and frequency (%), using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
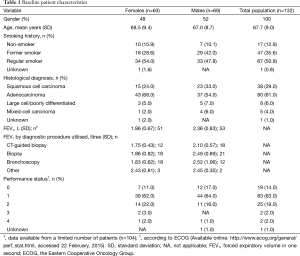
A total of 132 patients (mean age 67.7 years, 48% women) who met the inclusion criteria of a recorded diagnosis of an inoperable, malignant, bronchial tumour were identified in medical records (Table 1). Smoking history was recorded for all but one patient, and the study population included 86% current or former smokers. Of these, more females were current smokers, while men dominated the former smoker group (Table 1). Adenocarcinoma, diagnosed in 61% of the patients, was the most commonly recorded histological subtype, followed by squamous cell carcinoma in 29% of the patients (Table 1). Chronic obstructive pulmonary disease was the most commonly recorded co-morbid diagnosis in patients (17%), followed by hypertension (10%), diabetes mellitus (9%), ischaemic heart disease (9%), heart failure (8%), and other types of cancer (5%) (data not shown).
Full table
Lung function (measured by FEV1) showed mean FEV1 volumes close to or >2 L (Table 1). Also, the Eastern Cooperative Oncology Group (ECOG) performance status of the patients included in the study population was good overall, with 96% of patients having a value of ≤2 (Table 1).
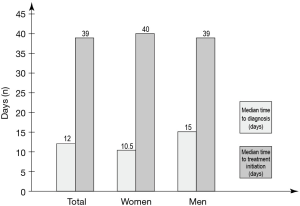
Most patients were examined using more than one diagnostic procedure, with 29% having been examined using CT-guided biopsy (Figure 1). Of all patients who underwent CT-guided biopsy, 39% were women and 61% were men. Overall, 63 patients were recorded to have received their diagnosis following a bronchoscopy and 25 patients were recorded to have received their diagnosis following a CT-guided [transthoracic core needle (TCN)] biopsy. The median time from initial hospital contact at an emergency or pulmonology clinic to an established diagnosis was 10.0 and 28.0 days, respectively, for bronchoscopy and CT-guided (TCN) biopsy. The overall median time to diagnosis from first contact for the whole population (limited data; 119 patients), irrespective of diagnostic procedure used, was 12.0 days. When divided by gender, females had a median time to diagnosis of 10.5 days compared to men who had 15.0 days (Figure 2).

No major differences were observed in the variables included for patients examined using CT-guided biopsy compared to the other procedures investigated. Patients who underwent a CT-guided biopsy examination were similar in age [68.4 (9.1) years] and had a similar lung function, measured by FEV1 [1.75 (0.43) L for women and 2.10 (0.57) L for men, and performance status (97.4% with ≤2)] as the total study population. Moreover, when patient data were analysed according to the predefined algorithm that included six important variables used to categorize patients suitable for a TCN biopsy, there was no difference between the overall study population (where data were available) and patients who actually underwent a CT-guided biopsy procedure (Table 2).
Full table
Overall, the most commonly unfulfilled algorithm criteria were normal P-PK and P-APTT, reported for 61% of the patients.
Complications following the three diagnostic procedures were reported for 11 patients overall, with a total of 13 reported incidents (six pneumothorax, five bleeding, one tachycardia and one dyspnoea). Only the pneumothorax complications led to un-planned, overnight hospital stays.
Median time to first-line treatment initiation from patient’s initial healthcare contact due to symptoms of lung cancer was 39.0 days, as reported for 129 of the total 132 patients included in the study. Three patients with negative time to first-line treatment were omitted from the calculation. No differences were observed when analysing time to treatment by gender (Figure 2).
Histopathology was the dominant technique used in 70% of patients to subtype and diagnose the disease, whilst the remaining 30% of patients underwent cytological testing. The majority of patients who underwent only bronchoscopy (66%) were diagnosed based on cytology. The mean percentage of tumour cells in the samples used for histopathology was 25.7% (based on data reported for 55 of the 132 patients). Testing for EGFR mutations was recorded in 26.5% of the cases, with a median time from first contact to mutation testing result of 98 days.
Within one year after a diagnosis of lung cancer, 60% (n=79) of patients in this population had died, and a further 20% (n=27) patients had records of progressive disease. The disease showed no signs of progression in the remaining 20% of patients.
Discussion
In this retrospective, descriptive study on the techniques used to obtain tumour samples from patients with advanced, inoperable NSCLC and their subsequent testing, the CT-guided biopsy frequency was shown to be fairly low and that the time to both diagnosis and initiation could be improved. The results are clinically relevant and provide a real-life description of the situation at regular hospitals in Sweden, with inclusion of all eligible patients in consecutive order (no patient selection).
The baseline pulmonary function in patients who may undergo a TCN biopsy procedure is important because it may be associated with pneumothorax, a procedure-related complication (15,16). In this population, the mean baseline lung function (FEV1) was similar to that reported previously for such patients (17) and did not appear to have been a discriminating factor for CT-guided biopsy. Patients who underwent CT-guided biopsy had similar FEV1 levels to the total population and, when considering the predefined algorithm, no differences were seen between patients who underwent either bronchoscopies or biopsies, including the CT-guided technique. Many CT-guided biopsies were performed despite the difficulties indicated in the pre-defined algorithm, suggesting that physicians were more willing to perform CT-guided biopsies than had been anticipated theoretically, possibly because the importance of a correct diagnosis outweighs the few risks associated with the procedure. Also, the most common deviation from the algorithm seen in the study (i.e., abnormal PK and APTT) could be handled, for example, by prospective withdrawal of anti-thrombotic treatments.
The study was conducted in 2009–10, during which targeted therapies, e.g., gefitinib, were introduced to Europe (11,13). Traditionally, lung cancer treatment decisions had been driven by histological typing and stage at diagnosis, but new tumour biomarkers and targeted therapies have raised the importance of molecular testing [8-10]. Targeted treatments require molecular testing and depend on obtaining an increased quantity and quality of tumour samples (3,7,9).
Overall, the specific technique used to obtain a tumour sample depended on the respective clinics/hospitals in the study with no apparent uniformity. This is probably related to local processes rather than lack of clear, national guidelines and reflects the complexity of diagnosing fragile patients with lung cancer.
Some patients underwent all three, main biopsy procedures (i.e., bronchoscopy, biopsy and CT-guided biopsy), whilst others only underwent a bronchoscopy, which was the most commonly performed technique (79% of all patients). Bronchoscopies are usually performed after initial symptomatic staging and imaging and are mostly diagnostic in patients whose lesions lie near bronchi (16,18-20). However, 66% of patients who only underwent bronchoscopy were diagnosed based on cytology, which may be insufficient for a molecular diagnosis that depends on both a high quantity and quality of actual tumour samples.
A TCN biopsy is a procedure which, although safe, is associated with an increased number of complications, such as pneumothorax, air embolism and bleeding, compared to other diagnostic techniques (16,18,20-22). An increased risk of pneumothorax has been reported when the biopsied lesion is small or when emphysema lies in the path of the biopsy needle; reduced lung function pre-biopsy or emphysema that lay in the path of the biopsy needle increased the need for chest-tube treatment of pneumothorax (21). Nevertheless, one conclusion was that the CT-guided core biopsy procedure is safe and applicable in a county hospital (21). In the present study, procedure-related complications from bronchoscopy, biopsy and CT-guided biopsy were observed in 11 patients and occurred most commonly following CT-guided biopsy, where five patients required extra night(s) of hospitalization due to pneumothorax.
The risk-benefit ratio of receiving a correct diagnosis based on molecular techniques seems reasonable given the prospect of finding a targeted treatment for patients. Further studies to assess whether there are any other predisposing “risk factors” for these complications, such as gender, lung function or other co-morbidities, and that might be used to screen patients suitable for TCN biopsies would be valuable.
Delays in diagnosis and treatment initiation may negatively affect a lung cancer patient’s psychological well-being (23). Delays between the imaging and biopsy step of tumour staging may also lead to growth in tumour size making them unsuitable for biopsy procedures (24). In this study, the overall median time to diagnosis from first patient contact with a physician was 12.0 days and longest in CT-guided biopsy patients (28.0 days), but shortest for bronchoscopy patients (10.0 days). Similarly, time to treatment initiation was approximately one month (median 39 days) in the overall patient population and longest in patients who had undergone a CT-guided biopsy. One reason for delays in the diagnosis and treatment initiation with CT-guided biopsies may be that bronchoscopies are often performed early in the patient work up, followed by a second TCN biopsy, e.g., a CT-guided biopsy, if the bronchoscopy was insufficient for diagnosis/treatment assessment (16,18-20). In Sweden, the recommendation is to have a treatment decision for 80% of patients within 28 days from arrival of the referral, but this is rarely followed. Nationally, this time is actually about 60 days (25). In this study, it took 64 days before 75% of patients had started first treatment from first contact with health care.
Today, patient diagnosis involves testing of several tumour biomarkers (8-11). At the time of this study, this diagnostic strategy was not fully developed because, for example, TKIs were not wholly established as the drug of choice for patients with EGFR mutations. In this study, only 35 out of 132 patients underwent EGFR mutation tests, the results of which took approximately three months to obtain. This time span suggests that these patients had already initiated first-line chemotherapy before being tested for EGFR mutational status to determine second- or third-line therapy. Since guidelines today highlight both testing of EGFR mutation status and the use of TKIs as first-line therapy for EGFR mutation positive patients (4), this could imply that patients in this study population who harboured an EGFR mutation did not, in fact, receive optimal first-line treatment. The situation in this study may reflect the novelty of TKIs and tumour marker techniques in Sweden during the investigated time period. The need for molecular tests for tumour biomarkers is now clear and currently a higher proportion of patients are likely to be tested first line (3,8,10).
As with all retrospective, observational medical record studies, this study may have limitations, and data retrieval is limited to the variables recorded. The data presented were collected at three selected centres and may not have provided a full national representation. However, all centres were specialist lung cancer clinics at three major university/county hospitals from different regions in Sweden, thus providing a sample of the lung cancer healthcare system. The study centres recruited approximately equal numbers of patients. The retrospective data collection from medical records ensured that no patient selection issues influenced the diagnosis, biopsy procedures or treatment choice, and caused the least possible inconvenience to patients.
This study reflects previous reports on the population and demographics of lung cancer patients (25), and the numbers of CT-guided biopsies and EGFR mutational tests performed can be considered as being low. Today, EGFR mutational testing for TKI-sensitive tumours is more common, as probably is the use of CT-guided biopsy for obtaining tumour samples, particularly as changes in, for example, needle bores may be associated with fewer procedure-associated complications (e.g., pneumothorax). In 2012, 40% of patients were tested for EGFR mutations nationally (25). Improved routines and large numbers of biopsies performed that increase experience might also lower the risk of complications. The times to diagnosis and treatment initiation are long and could be improved. A growing awareness of the importance of increasingly required collaboration between pulmonologists, radiologists and pathologists in the diagnosis and treatment of lung cancer together with personalized therapy using new, molecularly-targeted drugs (e.g., gefitinib and erlotinib) and its earlier diagnosis may improve the clinical outcomes of patients with lung cancer in Sweden.
Conclusions
Late-stage lung cancer is usually terminal. Its efficient, timely and accurate diagnosis is fundamental in determining the optimal, targeted treatment and personalizing therapy. This depends on an ever-increasing array of cytological, histological and molecular tests that require adequate tissue of good quality for analysis. TCN biopsies (e.g., CT-guided core needle biopsies) are relatively safe and well-established methods of obtaining good yield tumour samples, particularly for EGFR mutation status tests. These techniques may need to be further utilized following imaging for fast and reliable diagnoses based on histopathological and mutational analyses. The future diagnosis of lung cancer, therefore, will require a greater collaboration between surgeons, pulmonologists, radiologists and pathologists, leading to more personalized therapeutic strategies with targeted therapies, such as gefitinib or erlotinib.
Improved diagnostic routines according to the latest recommendations may lead to CT-guided biopsies being performed earlier and faster. With increased experience, this may ultimately improve outcomes in patients with advanced lung cancer.
Acknowledgements
This work was sponsored by AstraZeneca. Christina Ehrenkrona is a Biostatistician employed by TFS, Lund, Sweden and provided statistical analysis support funded by AstraZeneca. Dr. Grażyna Söderbom from Klipspringer AB provided medical writing support funded by AstraZeneca.
Footnote
Conflicts of Interest: Hirsh Koyi, Sven Nyrén and Leif Johansson have received financial compensation for their work. Jesper From is a full-time employee at AstraZeneca.
References
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32:605-44. [PubMed]
- Schabath MB, Thompson ZJ, Gray JE. Temporal trends in demographics and overall survival of non-small-cell lung cancer patients at Moffitt Cancer Center from 1986 to 2008. Cancer Control 2014;21:51-6. [PubMed]
- Jung CY. Biopsy and mutation detection strategies in non-small cell lung cancer. Tuberc Respir Dis (Seoul) 2013;75:181-7. [PubMed]
- Socialstyrelsen. Nationella riktlinjer för lungcancervård 2011 – stöd för styrning och ledning. Available online: http://www.socialstyrelsen.se/publikationer2011/2011-3-2, accessed 22 February, 2015.
- Socialstyrelsen. Cancerincidens i Sverige 2012 – Nya diagnosticerade cancerfall år 2012. Available online: http://www.socialstyrelsen.se/publikationer2013/2013-12-17, accessed 22 February, 2015.
- Koyi H, Hillerdal G, Brandén E. A prospective study of a total material of lung cancer from a county in Sweden 1997-1999: gender, symptoms, type, stage, and smoking habits. Lung Cancer 2002;36:9-14. [PubMed]
- Collins LG, Haines C, Perkel R, et al. Lung cancer: diagnosis and management. Am Fam Physician 2007;75:56-63. [PubMed]
- Domvri K, Zarogoulidis P, Darwiche K, et al. Molecular Targeted Drugs and Biomarkers in NSCLC, the Evolving Role of Individualized Therapy. J Cancer 2013;4:736-54. [PubMed]
- Kulesza P, Ramchandran K, Patel JD. Emerging concepts in the pathology and molecular biology of advanced non-small cell lung cancer. Am J Clin Pathol 2011;136:228-38. [PubMed]
- Ma PC. Personalized targeted therapy in advanced non-small cell lung cancer. Cleve Clin J Med 2012;79 Electronic Suppl 1:eS56-60.
- Araki T, Yashima H, Shimizu K, et al. Review of the treatment of non-small cell lung cancer with gefitinib. Clin Med Insights Oncol 2012;6:407-21. [PubMed]
- Chen X, Liu Y, Røe OD, et al. Gefitinib or erlotinib as maintenance therapy in patients with advanced stage non-small cell lung cancer: a systematic review. PLoS One 2013;8:e59314. [PubMed]
- Douillard JY, Ostoros G, Cobo M, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer 2014;110:55-62. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Heyer CM, Reichelt S, Peters SA, et al. Computed tomography-navigated transthoracic core biopsy of pulmonary lesions: which factors affect diagnostic yield and complication rates? Acad Radiol 2008;15:1017-26. [PubMed]
- Manhire A, Charig M, Clelland C, et al. Guidelines for radiologically guided lung biopsy. Thorax 2003;58:920-36. [PubMed]
- Win T, Jackson A, Sharples L, et al. Relationship between pulmonary function and lung cancer surgical outcome. Eur Respir J 2005;25:594-9. [PubMed]
- Anderson JM, Murchison J, Patel D. CT-guided lung biopsy: factors influencing diagnostic yield and complication rate. Clin Radiol 2003;58:791-7. [PubMed]
- Laroche C, Fairbairn I, Moss H, et al. Role of computed tomographic scanning of the thorax prior to bronchoscopy in the investigation of suspected lung cancer. Thorax 2000;55:359-63. [PubMed]
- McSweeney SE, O’Regan KN, Mc Laughlin PD, et al. Evaluation of the efficacy and safety of percutaneous biopsy of lung. Open Respir Med J 2012;6:82-8. [PubMed]
- Brandén E, Wallgren S, Högberg H, et al. Computer tomography-guided core biopsies in a county hospital in Sweden: Complication rate and diagnostic yield. Ann Thorac Med 2014;9:149-53. [PubMed]
- Yeow KM, Su IH, Pan KT, et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 2004;126:748-54. [PubMed]
- Koyi H, Hillerdal G, Brandén E. Patient’s and doctors’ delays in the diagnosis of chest tumors. Lung Cancer 2002;35:53-7. [PubMed]
- O’Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol) 2000;12:141-4. [PubMed]
- Nationella lungcancerregistret. Lungcancer, Nationell kvalitetsrapport för diagnosår 2012 från Nationella lungcancerregistret (NLCR). Available online: http://www.cancercentrum.se/globalassets/cancerdiagnoser/lunga-och-lungsack/vardprogram/natvp_lungcancer_2015-03-10.pdf, accessed 3 March, 2015.