Meta-analysis of the efficacy and safety of adding an angiotensin receptor blocker (ARB) to a calcium channel blocker (CCB) following ineffective CCB monotherapy
Introduction
Hypertension is a major risk factor for cardiovascular morbidity and mortality (1-4) and accounts for 13.5% of all-cause mortality worldwide. Strict blood pressure control, as recommended by professional guidelines, significantly reduces the risk of cardiovascular events, stroke and death (5,6). However, at present, the percentage of hypertensive patient achieving recommended targets is below 35% (7).
Guidelines for the management of hypertension (8,9) recommend that using initial monotherapy for treatment-naïve patients with addition of a second drug if monotherapy is ineffective. Calcium channel blockers (CCBs) are among recommended first-line treatment options. Advantageous pleiotropic effects of CCB treatment include slowing of atherosclerosis progression (10), improvements of cardiac (11) and renal function (12), reduction of diabetes risk (13). Treatment with CCB is associated with improved prognosis (14-16). However, 75% of hypertensive patients fail to reach the recommended blood pressure targets with monotherapy, and eventually require combination therapy (17). Prior studies demonstrated that adding an angiotensin receptor blocker (ARB) to CCB after initial ineffective CCB monotherapy, is well tolerated and can improve the percentage of patient achieving good blood pressure control (18). However, large-scale, randomized, controlled studies, evaluating the clinical benefits of converting to ARB + CCB following ineffective CCB monotherapy, are lacking.
We therefore conducted this meta-analysis to systematically review and analyze the clinical benefits of ARB + CCB following ineffective CCB monotherapy.
Methods
Literature search and retrieval
Two authors searched relevant studies published in PubMed until August 2015 using Medical Subject Headings (Mesh) and keyword searches. For Mesh, the search format was “Calcium Channel Blockers” [Mesh], “Angiotensin II Type 1 Receptor Blockers” [Mesh], and “Hypertension” [Mesh]; and the keywords were nifedipine, felodipine, amlodipine, lacidipine, nimodipine, nitrendipine, nicardipine, diltiazem, verapamil, calcium channel blockers, as well as losartan, valsartan, olmesartan, telmisartan, candesartan, irbesartan, angiotensin receptor inhibitor. In addition, we verified the references in the papers retrieved and included those that met the inclusion criteria in this meta-analysis.
Literature screening
The literature was screened according to the following criteria: (I) study design: randomized controlled trials (RCTs); (II) study population: hypertensive patients with ineffective CCB monotherapy; (III) treatment: CCB, with or without ARB; (IV) prognosis evaluation: improvement of (systolic or diastolic) blood pressure, blood pressure response rate, on-target rate of hypertension treatment, and the incidence of adverse events in hypertension patients receiving different antihypertensive treatments.
Data extraction
Two authors independently extracted the following data: lead author, year of publication, the characteristics of the population enrolled, the number of patients enrolled, study design, the experimental group [number of patients, drug(s) and dose(s)], the control group [number of patients, drug(s) and dose(s)], treatment time, definition of blood pressure, blood pressure response rate, the rate of normal blood pressure and related definition, and the incidence of adverse events and related definitions. Extracted data were entered into a standard EXCEL file (Microsoft Corp.) and were checked by another author of this paper. During data extraction, any discrepancy was resolved through discussions between the authors of this paper.
The primary efficacy endpoint of the studies was normal rate of blood pressure, the secondary efficacy endpoints were the response rate and change in blood pressure from baseline. The safety endpoint of the studies was incidence of adverse events.
Quality evaluation
After selection, the same two authors assessed the included RCTs independently for risk of bias using the Cochrane risk-of-bias tool (19). We assigned values of low, unclear or high risk of bias to the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other biases. Disagreements were resolved by consensus.
Statistical analysis
Differences are expressed as relative risks (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes and weighted mean differences (WMDs) with 95% CIs for continuous outcomes. Heterogeneity across studies was tested by using the I2 statistic, which is a quantitative measure of inconsistency across studies. Studies with I2 statistics of 25–50% were considered to have low heterogeneity, those with I2 statistics of 50–75% were considered to have moderate heterogeneity, and those with I2 statistics of 75% or greater were considered to have high heterogeneity (20). A fixed effect model was used regardless of heterogeneity. A P value <0.05 was considered statistically significant, except where otherwise specified. All statistical analyses were performed using Review Manager (Rev Man version 5.2; Nordic Cochrane Centre, Cochrane Collaboration).
IRB approval
This study used publicly available information and was therefore exempt from IRB approval.
Results
Study selection
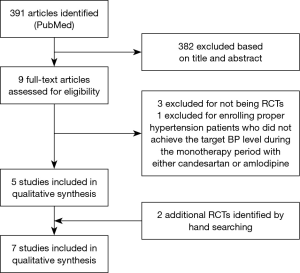
A total of 391 papers were retrieved, of which 382 papers were excluded based on review of title and abstract. Four papers were subsequently excluded based on full text review (21-24) [three papers (21,23,24) lacked a control group, and one study (22) did not enroll patients with ineffective CCB treatment]. Two papers were added after manual search and retrieval (25,26). Therefore, a total of 7 studies were included in this meta-analysis (25-31) (Figure 1).
Characteristics of selected studies
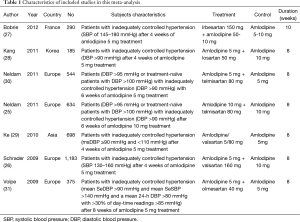
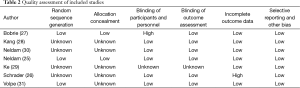
The characteristics of the 7 RCTs included are shown in Table 1. These RCTs were published in 2009-2012, with sample sizes of 185 to 1,183 subjects (total: 3,909 subjects). Six (25-27,29-31) of the seven included studies provided the percentage of on-target treatment before and after randomized treatment, six studies (25-30) provided blood pressure response rates before and after randomized treatment, and all seven studies (25-31) reported information about adverse events. The quality ratings of the selected studies are shown in Table 2.
Full table
Full table
Primary efficacy endpoint: percentage of on-target hypertension treatment
Of the six studies that provided the percentage of on-target hypertension treatment, the Neldam-2011 (30) and Volpe-2009 (31) studies included four subgroups, and we extracted only information about the amlodipine 5 mg group and the amlodipine 5 mg + telmisartan 80 mg group for our analysis. We defined target blood pressure as BP <140/90 mmHg.
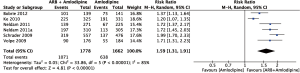
Figure 2 summarizes the result of the on-target rate of hypertension treatment using a random effects model. This meta-analysis included a total of 3,440 subjects, including 1,662 subjects in the amlodipine group and 1,778 subjects in the amlodipine + ARB group. The results showed that the on-target rate of hypertension treatment was significantly higher in the amlodipine + ARB group than in the amlodipine monotherapy group (RR =1.59; 95% CI, 1.31–1.91; P<0.01). There was significant heterogeneity among the six studies (I2=0.85).
Secondary efficacy endpoint: blood pressure response rate and changes in blood pressure
Blood pressure response rate
Of the six studies that provided blood pressure response rates, the Neldam-2011 (30) study included four subgroups; we extracted only information about the amlodipine 5 mg group and the amlodipine 5 mg + telmisartan 80 mg group for our analysis. We performed a meta-analysis of the response rates of systolic and diastolic blood pressure (DBP), where the response of systolic blood pressure (SBP) to drug treatment was defined as SBP ≤140 mmHg and the response of DBP to drug treatment was defined as DBP ≤90 mmHg or decrease of DBP ≥10 mmHg after randomization.
Four studies (25,27,28,30) reported the response rate of SBP. Figure 3A summarizes the response rate of SBP using a random effects model. This analysis included a total of 1,604 subjects, including 792 subjects in the amlodipine group and 812 subjects in the amlodipine + ARB group. The response rate of SBP was significantly higher in the amlodipine + ARB group than in the amlodipine monotherapy group (RR =1.28; 95% CI, 1.04–1.58; P<0.01). There was significant heterogeneity among the five studies (I2=0.85).
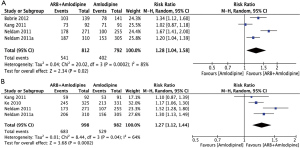
Four studies (25,28-30) reported the response rate of DBP. Figure 3B summarizes the response rate of DBP using a random effects model. This analysis included a total of 1,980 subjects, including 982 subjects in the amlodipine group and 998 subjects in the combination therapy group. Compared with amlodipine monotherapy, the response rate of DBP was significantly higher in hypertension patients receiving amlodipine + ARB (RR =1.27; 95% CI, 1.12–1.44; P=0.04). There was significant heterogeneity among the four studies (I2=0.64).
Changes in blood pressure
Of the 7 studies included, 3 studies (27-29) reported changes in blood pressure before and after randomized treatment. Figure 4A,B summarizes the change of SBP and DBP after randomization. The two analyses included a total of 545 subjects, including 283 subjects in the amlodipine and 272 subjects in the combination therapy group.
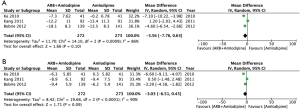
Figure 4A showed that, compared with amlodipine monotherapy, the change in SBP was higher in hypertensive patients receiving amlodipine + ARB (RR =−3.56; 95% CI, −7.76–0.63), but the difference did not reach statistical significance (P=0.10). There was significant heterogeneity among the three studies (I2=0.86). Figure 4B showed that, compared with amlodipine monotherapy, the delta DBP was higher in hypertensive patients receiving amlodipine + ARB (RR =−3.03; 95% CI, −6.51–0.45), but the difference did not reach statistical significance (P=0.09). There was significant heterogeneity among the three studies (I2=0.86).
Primary safety endpoint: adverse events
Figure 5 summarizes the results of adverse events among the different studies. A fixed effects model was used to compile the results because heterogeneity was small among the 7 studies (I2=0.09). ARB + amlodipine treatment carried a lower risk of adverse events relative to amlodipine monotherapy (RR =0.88; 95% CI, 0.80–0.96; P<0.01).

Discussion
This meta-analysis of 7 RCTs evaluated the improvement in blood pressure control and safety of CCB combined with ARB following ineffective CCB monotherapy. The results show that for patients with ineffective CCB monotherapy, adding an ARB significantly reduced blood pressure and significantly improved the response rate and the percentage of on-target hypertension treatment. In addition, for patients with ineffective CCB monotherapy, converting to CCB combined with ARB significantly reduced the incidence of adverse events relative to continued CCB monotherapy.
Many clinical studies and reviews have reported that for patients with ineffective CCB monotherapy, converting to CCB combined with ARB was safe and effective. The present study is the first meta-analysis of such studies. To ensure quality, this meta-analysis included only RCTs and excluded cohort studies.
Consistent with previous studies, this meta-analysis showed that CCB combined with ARB improved blood pressure control in patients with ineffective CCB monotherapy. Previous cohort studies (18) showed that for patients with ineffective CCB monotherapy, converting to CCB combined with ARB improved blood pressure control without increasing the incidence of adverse events; however, these studies did not include a control group and, thus, were unable to reach a definitive conclusion about whether CCB combined with ARB was superior to CCB monotherapy. Several RCTs (32-35) assigned treatment-naïve hypertension patients directly to the CCB monotherapy group or to the CCB combined with ARB group without having an initial screening period with CCB monotherapy; thus, these studies reached the conclusion that CCB combined with ARB was superior to CCB monotherapy in reducing blood pressure, but were unable to clarify whether CCB combined with ARB may improve blood pressure in patients with initial ineffective CCB monotherapy. In this meta-analysis, all selected RCTs enrolled patients with initial ineffective CCB monotherapy who were randomly assigned to the combination therapy group or to the monotherapy group. Therefore the results of this meta-analysis specifically demonstrate adding ARB to CCB improved blood pressure control in patients with ineffective initial CCB monotherapy.
Regarding safety, this meta-analysis showed that CCB combined with ARB significantly reduced the incidence of adverse events relative to CCB monotherapy. In addition to improvements in blood pressure, safety benefits were also derived from other clinical benefits independent of improvements in blood pressure. Previous studies have shown that for hypertensive patients whose blood pressure is 115/75–185/115 mmHg, the risk of death related to cardiovascular complications doubles with each increase of 20 mmHg in SBP or each increase of 10 mmHg in DBP (36); therefore, reducing blood pressure can significantly improve the prognosis of patients with cardiovascular and cerebrovascular diseases. Further, studies have shown that in addition to blood pressure-lowering, patients also benefit from pleiotropic effect during ARB and CCB treatment. ARBs are associated with cardiac and renal protective and reduced incidence of stroke (37,38). CCBs have anti-atherosclerotic effects and reduce the incidence of cardiovascular and cerebrovascular events (14). Studies have shown that ARB combined with CCB reduced the incidence of cardiovascular and cerebrovascular events (39-41). The results of this meta-analysis are consistent with the data from previous studies.
This meta-analysis included only RCTs, which, to a certain extent, ensured rigorous analysis. Nevertheless, this meta-analysis has some limitations. (I) The drug in CCBs group is only amlodipine, whether other kinds of CCBs have the same function remains unknown. (II) The studies included were characterized by different treatment durations, which may affect treatment outcome. (III) Different doses of ARB and CCB were used for combination therapy; therefore, the safety and effectiveness of different doses needs further validated. (IV) Different ARBs and CCBs were used in different studies, which increased the level of heterogeneity in this meta-analysis. However, the number of eligible RCTs would be greatly limited if only RCTs using a particular ARB or CCB were included. Thus, more clinical studies are needed to evaluate the efficacy of combination therapy using specific ARB(s) or CCB(s). (V) Studies have shown that a fixed formulation of ARB + CCB was superior to one ARB (randomly) combined with one CCB (42). Among the studies included in this meta-analysis, six studies used a fixed formulation of ARB + CCB, and four studies used one ARB (randomly) combined with one CCB. We did not compare the treatment outcomes between these two combination regimens. (VI) Regarding safety, this meta-analysis only analyzed adverse events and did not analyze the effects of combination therapy on blood chemistry.
Future research should further investigate the following topics to provide a stronger basis for precise diagnosis and treatment of hypertension. (I) The safety and efficiency of ARB(s) + CCB(s) therapy using different doses, different treatment durations, and different drugs should be evaluated. (II) The advantages of fixed formulation(s) of ARB + CCB over one ARB (randomly) combined with one CCB should be explored. (III) The safety and efficiency of ARB + CCB in high-risk and medium-to-low-risk patients, different races, different age groups, and different genders should be evaluated. (IV) In addition to adverse events, the safety of ARB + CCB should also be evaluated based on blood chemistry. (V) Studies have shown that ARB + CCB reduced the risk of cardiovascular events and the incidence of stroke, and future studies should evaluate cardiovascular and cerebrovascular benefits in addition to improved blood pressure.
Conclusions
The results of our meta-analysis demonstrate that adding an ARB to CCB after initial ineffective CCB monotherapy, significantly improved blood pressure control and the percentage of on-target hypertension treatment with significantly reduced incidence of adverse events compared with continued CCB monotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lawes CM, Vander Hoorn S, Rodgers A, et al. Global burden of blood-pressure-related disease, 2001. Lancet 2008;371:1513-8. [PubMed]
- Kannel WB. Elevated systolic blood pressure as a cardiovascular risk factor. Am J Cardiol 2000;85:251-5. [PubMed]
- Kannel WB. Historic perspectives on the relative contributions of diastolic and systolic blood pressure elevation to cardiovascular risk profile. Am Heart J 1999;138:205-10. [PubMed]
- Yu JM, Kong QY, Schoenhagen P, et al. The prognostic value of long-term visit-to-visit blood pressure variability on stroke in real-world practice: a dynamic cohort study in a large representative sample of Chinese hypertensive population. Int J Cardiol 2014;177:995-1000. [PubMed]
- Turnbull F; Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003;362:1527-35. [PubMed]
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665.
- Cutler JA, Sorlie PD, Wolz M, et al. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999-2004. Hypertension 2008;52:818-27. [PubMed]
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507-20. [PubMed]
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159-219. [PubMed]
- Zanchetti A, Bond MG, Hennig M, et al. Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis: principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double-blind, long-term trial. Circulation 2002;106:2422-7. [PubMed]
- Wu L, Deng SB, She Q. Calcium channel blocker compared with angiotensin receptor blocker for patients with hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich) 2014;16:838-45. [PubMed]
- Tani S, Takahashi A, Nagao K, et al. Effects of the T/L-type calcium channel blocker benidipine on albuminuria and plasma aldosterone concentration. A pilot study involving switching from L-type calcium channel blockers to benidipine. Int Heart J 2014;55:519-25. [PubMed]
- Shimada K, Miyauchi K, Daida H. Azelnidipine and glucose tolerance: possible indications and treatment selection for hypertensive patients with metabolic disorders. Expert Rev Cardiovasc Ther 2015;13:23-31. [PubMed]
- Dahlöf B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005;366:895-906. [PubMed]
- Iwakura K, Ito H, Ishii K, et al. Changes in left ventricular relaxation after azelnidipine treatment in hypertensive patients with diabetes: subanalysis of a prospective single-arm multicentre study. BMJ Open 2014;4:e006136. [PubMed]
- Li X, Yang MS. Effects of T-type calcium channel blockers on renal function and aldosterone in patients with hypertension: a systematic review and meta-analysis. PLoS One 2014;9:e109834. [PubMed]
- Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med 2009;122:290-300. [PubMed]
- Allemann Y, Fraile B, Lambert M, et al. Efficacy of the combination of amlodipine and valsartan in patients with hypertension uncontrolled with previous monotherapy: the Exforge in Failure after Single Therapy (EX-FAST) study. J Clin Hypertens (Greenwich) 2008;10:185-94. [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PubMed]
- Brachmann J, Ansari A, Mahla G, et al. Effective and safe reduction of blood pressure with the combination of amlodipine 5 mg and valsartan 160 mg in hypertensive patients not controlled by calcium channel blocker monotherapy. Adv Ther 2008;25:399-411. [PubMed]
- Maeda A, Tamura K, Kanaoka T, et al. Combination therapy of angiotensin II receptor blocker and calcium channel blocker exerts pleiotropic therapeutic effects in addition to blood pressure lowering: amlodipine and candesartan trial in Yokohama (ACTY). Clin Exp Hypertens 2012;34:249-57. [PubMed]
- Ohishi M, Kawai T, Hayashi N, et al. Effect of tablets with a combination of telmisartan and amlodipine on patients with hypertension: the Cotalo study. Hypertens Res 2013;36:620-6. [PubMed]
- Punzi H, Neutel JM, Kereiakes DJ, et al. Efficacy of amlodipine and olmesartan medoxomil in patients with hypertension: the AZOR Trial Evaluating Blood Pressure Reductions and Control (AZTEC) study. Ther Adv Cardiovasc Dis 2010;4:209-21. [PubMed]
- Neldam S, Edwards C, Jones R, et al. Switching patients with uncontrolled hypertension on amlodipine 10 mg to single-pill combinations of telmisartan and amlodipine: results of the TEAMSTA-10 study. Curr Med Res Opin 2011;27:2145-53. [PubMed]
- Schrader J, Salvetti A, Calvo C, et al. The combination of amlodipine/valsartan 5/160 mg produces less peripheral oedema than amlodipine 10 mg in hypertensive patients not adequately controlled with amlodipine 5 mg. Int J Clin Pract 2009;63:217-25. [PubMed]
- Bobrie G. I-COMBINE Study Investigators. I-COMBINE study: assessment of efficacy and safety profile of irbesartan/amlodipine fixed-dose combination therapy compared with amlodipine monotherapy in hypertensive patients uncontrolled with amlodipine 5 mg monotherapy: a multicenter, phase III, prospective, randomized, open-label with blinded-end point evaluation study. Clin Ther 2012;34:1705-19. [PubMed]
- Kang SM, Youn JC, Chae SC, et al. Comparative efficacy and safety profile of amlodipine 5 mg/losartan 50 mg fixed-dose combination and amlodipine 10 mg monotherapy in hypertensive patients who respond poorly to amlodipine 5 mg monotherapy: an 8-week, multicenter, randomized, double-blind phase III noninferiority study. Clin Ther 2011;33:1953-63. [PubMed]
- Ke Y, Zhu D, Hong H, et al. Efficacy and safety of a single-pill combination of amlodipine/valsartan in Asian hypertensive patients inadequately controlled with amlodipine monotherapy. Curr Med Res Opin 2010;26:1705-13. [PubMed]
- Neldam S, Lang M, Jones R, et al. Telmisartan and amlodipine single-pill combinations vs amlodipine monotherapy for superior blood pressure lowering and improved tolerability in patients with uncontrolled hypertension: results of the TEAMSTA-5 study. J Clin Hypertens (Greenwich) 2011;13:459-66. [PubMed]
- Volpe M, Brommer P, Haag U, et al. Efficacy and tolerability of olmesartan medoxomil combined with amlodipine in patients with moderate to severe hypertension after amlodipine monotherapy: a randomized, double-blind, parallel-group, multicentre study. Clin Drug Investig 2009;29:11-25. [PubMed]
- Destro M, Luckow A, Samson M, et al. Efficacy and safety of amlodipine/valsartan compared with amlodipine monotherapy in patients with stage 2 hypertension: a randomized, double-blind, multicenter study: the EX-EFFeCTS Study. J Am Soc Hypertens 2008;2:294-302. [PubMed]
- Flack JM, Calhoun DA, Satlin L, et al. Efficacy and safety of initial combination therapy with amlodipine/valsartan compared with amlodipine monotherapy in black patients with stage 2 hypertension: the EX-STAND study. J Hum Hypertens 2009;23:479-89. [PubMed]
- Smith TR, Philipp T, Vaisse B, et al. Amlodipine and valsartan combined and as monotherapy in stage 2, elderly, and black hypertensive patients: subgroup analyses of 2 randomized, placebo-controlled studies. J Clin Hypertens (Greenwich) 2007;9:355-64. [PubMed]
- Weber MA, White WB, Sica D, et al. Effects of combining azilsartan medoxomil with amlodipine in patients with stage 2 hypertension. Blood Press Monit 2014;19:90-7. [PubMed]
- Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903-13. [PubMed]
- Lindholm LH, Ibsen H, Dahlöf B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:1004-10. [PubMed]
- Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003;21:875-86. [PubMed]
- Kim-Mitsuyama S, Ogawa H, Matsui K, et al. An angiotensin II receptor blocker-calcium channel blocker combination prevents cardiovascular events in elderly high-risk hypertensive patients with chronic kidney disease better than high-dose angiotensin II receptor blockade alone. Kidney Int 2013;83:167-76. [PubMed]
- Koyanagi R, Hagiwara N, Yamaguchi J, et al. Efficacy of the combination of amlodipine and candesartan in hypertensive patients with coronary artery disease: a subanalysis of the HIJ-CREATE study. J Cardiol 2013;62:217-23. [PubMed]
- Sawada T, Yamada H, Dahlöf B, et al. Effects of valsartan on morbidity and mortality in uncontrolled hypertensive patients with high cardiovascular risks: KYOTO HEART Study. Eur Heart J 2009;30:2461-9. [PubMed]
- Baser O, Andrews LM, Wang L, et al. Comparison of real-world adherence, healthcare resource utilization and costs for newly initiated valsartan/amlodipine single-pill combination versus angiotensin receptor blocker/calcium channel blocker free-combination therapy. J Med Econ 2011;14:576-83. [PubMed]