Pulmonary lymphoepithelioma-like carcinoma: a Surveillance, Epidemiology, and End Results database analysis
Introduction
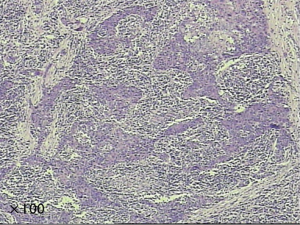
Lymphoepithelioma-like carcinoma (LELC) is one of the uncommon histological types of cancer. It is first reported as the Epstein-Barr virus associated malignancy in 1987 by Bégin et al. (1). It is usually found in pharyngeal tissue (2), while cases involving other organs have also been reported, such as lacrimal gland (3), cervix (4), bladder (5) and skin (6) etc. LELC is recognized as the diffused lymphoepithelial cells among the inflammatory tissue. In the past two decades, there are about 200 cases of pulmonary LELC have been reported in literature. It is reported that pulmonary LELC exhibits a better prognosis than other types of lung cancer among Asian patients (7-10). Complete resection is the standard treatment for early stage patients, while chemotherapy and radiotherapy also have effects to some extent in the last stage patients. The pathologic specimens demonstrated nests of epithelial tumor cells separated or infiltrated by numerous lymphocytes (Figure 1). In the previous version of WHO tumor classifications, LELC was a subtype of large cell lung cancer (LCC) (11,12). In the latest version of WHO classification, it has been categorized as the other and unclassified carcinoma (13). The majority of the studies are from Asian patients, there are only few cases have been reported in the western patients (14-16). Due to the low prevalence, the clinical characteristics and the prognosis of LELC remained unclear, especially among the western population.
Given the lack of the data on its prognosis and characteristics, we performed an analysis on the Surveillance, Epidemiology, and End Results database (SEER) to investigate the clinical characteristics, prognosis and risk factors of pulmonary LELC. Besides, we intended to compare the prognosis of LELC with other LCC, adenocarcinoma (AD) and squamous cell lung cancer (SQ) in order to verify the classification in the epidemiology aspect.
Materials and methods
Data source
The SEER program is supported by the National Cancer Institute. It was collected from 18 population-based cancer registered institutes that cover approximately 28% of US population. The present study was performed with the SEER public-access database. The duration of the study was set from 1973 to December 2012 which was the date of database record cut-off. However, the earliest records of pulmonary LELC were only available from 1993 in SEER database. SEER*Stat 8.2.1 software was utilized to extract the data from SEER database.
Search strategy and inclusion criteria
Referring to the western population, all patients who were diagnosed cancer with primary site of lung and bronchus from 1973 to December 2012 were identified with the ICD-0–3/WHO 2008 criteria in SEER database. The histology selection was limited to LELC, which was coded as 8082 according to the ICD-0–3 histology. Besides, the patients who were diagnosed other types of lung cancer during the same period were also identified. We extracted the data using case listing session of SEER*Stat 8.2.1 software. All patients were included in order to maximize the searching result.
Statistical analysis
Since the AJCC TMN staging was not applicable in this study. All the patients were categorized by summary stages (localized, regional and distant). Basic patient characteristics were compared using Kruskal-Wallis test for continuous variables i.e., age at diagnosis. While, chi-square test of Fisher’s exact test was utilized for categorical variables comparison i.e., gender and race. Variables including age at diagnosis, gender, race, grade, summary stage, CHSDA regions, primary site, surgery, and radiation were included in the survival analysis models. The overall survival (OS) was calculated from the date of diagnosis to the date of death or last follow-up. OS and other survival functions were estimated by the Kaplan-Meier (KM) method and the log-rank test was used to assess differences in OS stratified by each variable. Univariate and multivariate survival analyses were conducted using the Cox proportional hazards model. Multivariable analysis was conducted by entering age, race, gender, tumor size, and CHSDA region into the Cox proportional hazards model. The variables which had P value <0.5 in univariate analysis were put into the multivariate analysis. The model was stratified by summary stage since it was shown to be a time dependent covariate and by age of diagnosis.
The statistical analysis was performed using the software of SPSS 16.0 and GraphPad Prism 5. The statistical difference was considered as significant when P<0.05.
Results
Basic characteristics
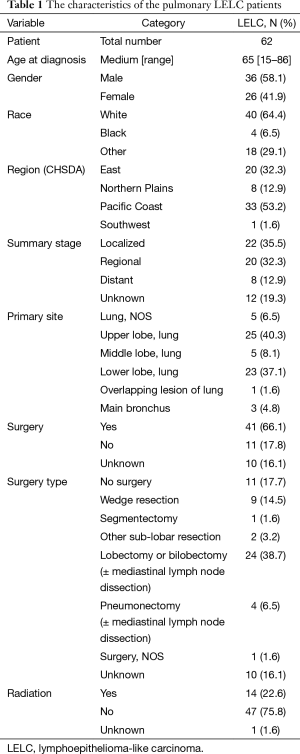
There were 790,301 cases of lung and bronchus cancer cases identified in SEER from 1973 to December 2012. Among of them, 62 cases of pulmonary LELC were identified and recorded. The median age at diagnosis was 65 ranging from 15–86. There were 36 cases of male and 26 cases of female in the included patients. White patients accounted for the largest proportion (N=40, 64.4%), while only four cases of black people were included. The rest of patients were other races including American Indian, Asian and Pacific Islander. These patients were mainly from Pacific Coast and East region (53.2% and 32.3%, respectively). Due to the fact that using American Joint Committee on Cancer (AJCC) 6th edition classification would greatly compromise data availability, the included cases were categorized by SEER historic classification as summary stage localized (tumor only in lung), regional (pulmonary and mediastinum lymph node involved) and distant (metastasis). Twenty-two patients were categorized as localized disease, while 20 and 8 patients with regional and distant disease, respectively. Regarding the primary site, upper lobe and lower lobe represented the majority of the disease which was 40.3% and 37.1%. Referring to treatments, only 14 patients underwent radiation therapy. Moreover, 66.1% patients received surgery. Among of them, 19.3% had sub-lobar resection including wedge resection and segmentectomy. A total of 45.2% received lobectomy, bilobectomy and pneumonectomy with or without mediastinum lymph node dissection (Table 1).
Full table
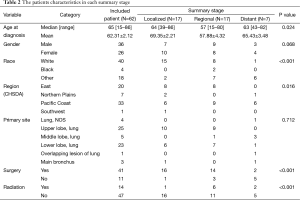
We investigated the characteristics of the patients who were categorized by the summary tumor stage. Patients with localized and distant disease were significantly older than those of regional disease. White patients and those who were from the pacific coast region had a greater possibility to suffer from the pulmonary LELC. There was no significant difference of gender. In terms of treatments, patients were less likely to receive radiation therapy. However, those who presented localized and regional disease mostly preferred surgery (Table 2).
Full table
Survival analysis
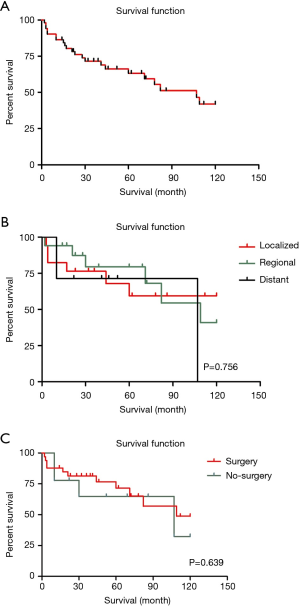
The OS rate by 1, 3 and 5 years of patients with LELC were 85.6%, 68.9% and 59.5%, comparing to 39.1%, 18% and 12.9% of other histopathological types of lung cancer. Besides, in each particular summary stage (localized, regional and distant), only 56 of 62 patients with complete survival data were included in this study. The median follow-up duration was 67 months. While, the median survival of all LELC patients was 107 months [95% confidence interval (CI), 67–147] which was much better than other non-LELC patients [median survival: 13 months (95% CI, 12.9–13.1)]. The KM curves of OS in the all LELC patients and each summary stage were demonstrated in Figure 2.
In the comparison between LELC and non-LELC, it was obvious that LELC had a better prognosis (Figure 3A). We were trying to study the difference of OS between LELC, LCC, AD and SQ. We found that LELC was superior to others (Figure 3B). Most of early stage LELC patients received surgery. Therefore, we compared the OS of postoperative patients between LELC and other types (LCC, AD and SQ). The result showed that LELC had better OS than LCC and SQ. However, no significant difference had been identified between LELC and AD (Figure 3C). In the non-surgery category, the OS of LELC patients was superior to other types of lung cancer patients (Figure 3D).
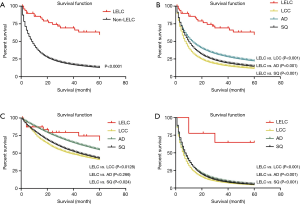
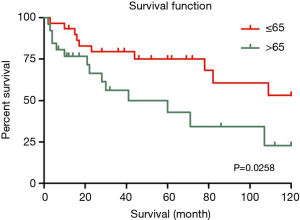
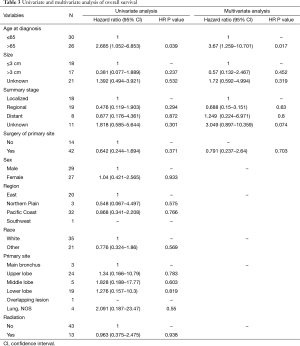
In the univariate analysis, race, region and gender of the patients were not associated with worse survival statistically (P values were 0.569, 0.766 and 0.933, respectively). However, younger patients (≤65-year old) had better survival outcomes than older patients (>65-year old) (HR 2.685, 95% CI, 1.052–6.853, P=0.039) (Figure 4). Referring to the stage, it seemed that late summary stage of LELC was not correlated to a worse outcome (Figure 3). The same result was shown in the primary site of tumor. In terms of treatment, there was no statistically difference between the patients whether had received radiation therapy or not (P=0.938). No significant difference had been found between surgery and no-surgery groups (HR 0.642, 95% CI, 0.244–1.694, P=0.371) (Table 3).
Full table
In the multivariate analysis, there was no prominent tendency towards worse survival outcome in groups of tumor size, surgery or summary stage except age. Patients who were older than 65 had worse prognostic outcomes (Table 3).
Discussion
Pulmonary LELC is a typically rare type of lung cancer. The majority of cases were reported in Asian population. There are very few cases reported in the western population. By analyzing the data from SEER, we intended to identify some clinical characteristics of western pulmonary LELC patients. We found that the prevalence of pulmonary LELC was very low. Due to the imitation of pathological techniques in the early years, it was only 62 cases available which had been recorded in SEER comparing to 790,239 cases of non-LELC.
According to our result, early stage patients (localized and regional) accounted for the largest proportion of LELC patients (67.8%). The age at diagnosis of the patients was ranging from 15 to 86. In the previous studies of Asian people, the median ages were reported as 51–55 (17-19). Whereas, we reported that the median age of the western patients were 65 which was older than the Asian patients. A total of 36 male patients and 27 female patients were included in our study. The male/female ratio was 1.33:1 which was consistent with the result of Chan’s (17) and Liang’s (19) studies (1.2:1 and 1.26:1, respectively). In this cohort, white patients accounted for the largest proportion (64.4%), which showed a consistency with the distribution of races in western population. Majority of the LELC patients were from Pacific Coast and East region (53.2% and 32.3%). It probably attributed to the advanced development in these regions, in which patients were likely more affordable to medical costs.
The prognosis of pulmonary LELC patients was better than other non-LELC patients. The median survival of LELC patients was 107 months, whereas the median survival of other non-LELC patients was only 13 months. Liang et al. reported that the 5-year survival rate of pulmonary LELC patients was 62% (19). Meanwhile, our study reported the 5-year survival rate of pulmonary LELC in western population was 59.5%, which is consistent with the results of previous studies regarding Asian population (7,14). It showed that the western pulmonary LELC patients also exhibited good prognosis. In the univariate and multivariate analysis, patients who were older than 65 had worse survival. However, the results demonstrated that the gender, human races, primary site of the tumor was not correlated to the OS of the pulmonary LELC. Moreover, the summary stage was not associated with the prognosis either. It indicated that patients with pulmonary LELC had good prognosis in each summary stage (Table 3).
In terms of treatments, there were 41 patients who had undergone surgical resections and 14 patients had received radiation therapy. It was clear that surgery was the first choice for the early stage LELC patients (37/45, 82.2%), while those who were in the late stage of pulmonary LELC preferred radiation instead of surgery (Table 2). We found no significant effect of surgery either in univariate or multivariate analysis. Theoretically, the late stage patients who were likely to receive non-surgical treatments had worse prognosis than early stages patients. However, LELC had a remarkably good prognosis that surgery might not bring enough benefit to the early stage patients to generate the advantages. On the other hand, previous studies reported that both early stage and advance stage of pulmonary LELC had ideal responses to chemotherapy (20,21). In our cohort, the advance stage LELC patients would have received chemotherapy which was able to prolong their OS as well as the surgical treatment to the early stage patients. Hence, no significant difference was observed in our cohort between surgery and no-surgery patients. Unfortunately, the data of chemotherapy was not available in SEER.
In the current version of WHO tumor classification, LELC was categorized as the other and unclassified carcinoma (13). However, few studies had clarified the clinical characteristics between LELC and other LCC. In this study, we found that LELC had a better prognosis than other LCC (Figure 3B). As a result, it showed that LELC was different from LCC both in morphology and epidemiology. Not surprisingly, it has been categorized as the other and unclassified carcinoma in the latest WHO classification (13).
There was no significant difference of OS between postoperative LELC and AD patients (Figure 3C). The possible reasons accounted for the insignificance were as follow: (I) majority of patients receiving surgery were in the early stage. These patients possessed good prognosis in each categories; (II) the follow-up time was insufficient to illustrate the superiority of LELC; (III) the sample size of LELC was smaller than others. The number of death would influence the survival rate more greatly in small sample size cohort. In consistent with the previous studies in Asian, it was clear that LELC patients had ideal prognosis after appropriate treatments.
According to our results, radiation did not bring any benefit to the pulmonary LELC patients regarding to OS (Table 3). It was well accepted that radiation was usually selected for unresectable patients or those with adverse condition which were related to poor survival and prognosis. Although our finding demonstrated that radiation brought no benefit in OS, it was unable to exclude the possibility that radiation was effective in prolong PFS. Unfortunately, SEER did not provide the any recurrence data.
Limitations have to be admitted in this study when utilizing the SEER database to explore such rare pathological type of lung cancer. Firstly, some individual data of the patients is not available such as smoking condition, performance status, gene mutation and details of lymph node situation. Chang et al. reported that only 17.4% of patients with lung LELCs possessed EGFR mutations, and Tam et al. observed that EGFR mutations were uncommon in LELC (7,21). Unfortunately, we were not able to perform such analysis with insufficient data. Secondly, some studies have reported that LELC is sensitive to capecitabine and docetaxel-based regimen (22,23), but it is difficult to study the response of LELC to the first-line or adjuvant chemotherapy without chemotherapy data. Additionally, it is impossible to evaluate the effect of treatments besides OS, because the individual recurrence situation and complications of treatments are not provided. Finally, there are 62 cases of pulmonary LELC have been identified in the SEER database. In terms of the survival data, only 56 of 62 cases have been recorded. Thus, the results of some analysis are not significant due to the small sample size. It would be more reliable and convincing if more data were available.
In conclusion, pulmonary LELC is also a rare type of lung cancer in the western population. In this cohort, the characteristics of LELC were consistent with the previous studies from Asia. Surgical resection is generally accepted in early stage LELC patients. No prognostic factor has been identified in our study. LELC patients have better prognosis than LCC, AD and SQ. Therefore, we suggest that the treatment of LELC should be well-considered. In order to understand pulmonary LELC more thoroughly, more cases are required.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bégin LR, Eskandari J, Joncas J, et al. Epstein-Barr virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol 1987;36:280-3. [PubMed]
- Iezzoni JC, Gaffey MJ, Weiss LM. The role of Epstein-Barr virus in lymphoepithelioma-like carcinomas. Am J Clin Pathol 1995;103:308-15. [PubMed]
- Blasi MA, Ventura L, Laguardia M, et al. Lymphoepithelioma-like carcinoma involving the lacrimal gland and infiltrating the eyelids. Eur J Ophthalmol 2011;21:320-3. [PubMed]
- Tseng CJ, Pao CC, Tseng LH, et al. Lymphoepithelioma-like carcinoma of the uterine cervix: association with Epstein-Barr virus and human papillomavirus. Cancer 1997;80:91-7. [PubMed]
- Williamson SR, Zhang S, Lopez-Beltran A, et al. Lymphoepithelioma-like carcinoma of the urinary bladder: clinicopathologic, immunohistochemical, and molecular features. Am J Surg Pathol 2011;35:474-83. [PubMed]
- Aoki R, Mitsui H, Harada K, et al. A case of lymphoepithelioma-like carcinoma of the skin associated with Epstein-Barr virus infection. J Am Acad Dermatol 2010;62:681-4. [PubMed]
- Chang YL, Wu CT, Shih JY, et al. Unique p53 and epidermal growth factor receptor gene mutation status in 46 pulmonary lymphoepithelioma-like carcinomas. Cancer Sci 2011;102:282-7. [PubMed]
- Han AJ, Xiong M, Gu YY, et al. Lymphoepithelioma-like carcinoma of the lung with a better prognosis. A clinicopathologic study of 32 cases. Am J Clin Pathol 2001;115:841-50. [PubMed]
- Ho JC, Wong MP, Lam WK. Lymphoepithelioma-like carcinoma of the lung. Respirology 2006;11:539-45. [PubMed]
- Ngan RK, Yip TT, Cheng WW, et al. Clinical role of circulating Epstein-Barr virus DNA as a tumor marker in lymphoepithelioma-like carcinoma of the lung. Ann N Y Acad Sci 2004;1022:263-70. [PubMed]
- Travis WD, Colby TV, Corrin B, et al, editors. Histological typing of lung and pleural tumours, World Health Organization International Histological Classification of Tumor. Berlin: Springer, 1999.
- Travis WD, Brambilla E, Müller-Hermelink HK, et al, editors. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC, 2004.
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [PubMed]
- Castro CY, Ostrowski ML, Barrios R, et al. Relationship between Epstein-Barr virus and lymphoepithelioma-like carcinoma of the lung: a clinicopathologic study of 6 cases and review of the literature. Hum Pathol 2001;32:863-72. [PubMed]
- Ferrara G, Nappi O. Lymphoepithelioma-like carcinoma of the lung. Two cases diagnosed in Caucasian patients. Tumori 1995;81:144-7. [PubMed]
- Morbini P, Riboni R, Tomaselli S, et al. Eber- and LMP-1-expressing pulmonary lymphoepithelioma-like carcinoma in a Caucasian patient. Hum Pathol 2003;34:623-5. [PubMed]
- Chan JK, Hui PK, Tsang WY, et al. Primary lymphoepithelioma-like carcinoma of the lung. A clinicopathologic study of 11 cases. Cancer 1995;76:413-22. [PubMed]
- Han A, Xiong M, Gu Y, et al. Clinicopathologic features and prognosis of lymphoepithelioma-like carcinoma of the lung. Zhonghua Bing Li Xue Za Zhi 2001;30:328-31. [PubMed]
- Liang Y, Wang L, Zhu Y, et al. Primary pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with long-term follow-up. Cancer 2012;118:4748-58. [PubMed]
- Huang CJ, Feng AC, Fang YF, et al. Multimodality treatment and long-term follow-up of the primary pulmonary lymphoepithelioma-like carcinoma. Clin Lung Cancer 2012;13:359-62. [PubMed]
- Tam IY, Chung LP, Suen WS, et al. Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res 2006;12:1647-53. [PubMed]
- Ho JC, Lam DC, Wong MK, et al. Capecitabine as salvage treatment for lymphoepithelioma-like carcinoma of lung. J Thorac Oncol 2009;4:1174-7. [PubMed]
- Ho JC, Lam WK, Wong MP, et al. Lymphoepithelioma-like carcinoma of the lung: experience with ten cases. Int J Tuberc Lung Dis 2004;8:890-5. [PubMed]