
Should psychological distress be listed as a surgical indication for indeterminate pulmonary nodules: protocol for a prospective cohort study in real-world settings
Introduction
Pulmonary nodules (PNs) are increasingly detected in the last decade owing to the extensive implementation of low-dose computed tomography (LDCT) for screening or diagnostic purposes in general population. Although less than 5% were malignant in nature (1), PNs were documented in as many as about 30% of computed tomography (CT) reports (2), leaving a considerable proportion of “indeterminate pulmonary nodules (IPN)”. Since IPN is accompanied with a potential risk of malignancy, adherence to a rigorous follow-up plan is usually required as per the current clinical recommendations (3). During the follow-up, the patients might need multiple CT scans and experience non-negligible psychological distress, such as anxiety or depression (4). The so-called “scanxiety” was significantly associated with impaired health-related quality of life (HRQoL) (4,5), especially in those who substantially over-estimated their risk of getting lung cancer (6). Patient-centered communication is therefore recommended to alleviate the undue distress and to achieve shared decision-making (7).
Substantial studies have been carried out to investigate the psychological burden in lung cancer screening recipients (4,7,8). Indeterminate or suspicious screening results were usually followed by a short-term increase of psychological burden to a clinically significant level (9,10). Although lung cancer-specific distress, as a whole, appeared to be resolved after a long-term follow (11), it can persist in specific patients with the perniciousness outweigh the benefit to prevent overtreatment by follow-up. To say the least, follow-up surveillance itself is not free from harmfulness, as cumulative exposure to radiation from recurrent CT scans was reported to increase the risk of lung cancer beyond that associated with cigarette smoking theoretically (12,13). This might, in return, exacerbate the psychological distress in certain patients. Therefore, severe anxiety or depression has been proposed, in the Chinese expert consensus, to be one of the surgical indications for IPN, leading to an important discrepancy in IPN patient management between China and Western countries (14). According to the expert consensus of China, surgical resection is postulated to mitigate the psychological distress in these patients, but the magnitude of resolution and its clinical significance are yet to be reported. There is no solid evidence that supports the abovementioned hypothesis, and thus psychological distress may sometimes result in the overtreatment of the benign or indolent nodules.
Various instruments were used to measure the psychological state among recipients of lung cancer screening, including the European Quality of Life (EQ-5D), the State-Trait Anxiety Inventory (STAI), the impact of event scale (IES), consequences of screening in Lung Cancer (COS-LC), as well as the Hospital Anxiety and Depression Scale (HADS) (4,7,8). Except for HADS, most of these scales are generic HRQoL measures which include dimensions of psychological outcomes. The HADS, a popular and valid instrument to assess clinical psychological morbidity in cancer patients, are less commonly applied in previous studies of PNs (15,16). For instance, the HADS was used in the UK Lung Screening Trial (UKLS) and the Lung Screen Uptake Trial (LSUT) to compare the screening and non-screening arms. The trials showed a temporary increase of anxiety in the screening patients, which was in consistence with other studies (17,18). Among the screening-recipients, those with IPN were shown to have the highest HADS-anxiety scores, with a mean score of 6.93 [95% confidence interval (CI): 5.65 to 8.21] (18). However, the two studies only longitudinally monitored the patients in follow-up. In real-world practice, IPN patients who are recommended for follow-up surveillance might request for surgery due to their fear of lung cancer development. So far, the change in psychological status following the IPN resection and the issuing of pathological report has not been reported. This prospective cohort study aims to identify the IPN patients with abnormal or borderline HADS score at baseline, and to compare changes in psychological burden in the patients with or without stress-driven surgery in real-world setting. The study results will answer whether severe psychological distress should be listed as surgical indication of IPN, and will demonstrate the feasibility of using HADS as a psychological screening tool in a thoracic clinic.
Methods
Design and setting
This proposed research is a prospective, observational study that aims to enroll 376 IPN patients with abnormal or borderline results of HADS measurement (see supplementary material, available at https://cdn.amegroups.cn/static/public/JTD-21-1423-Supplementary.pdf) at their first visit to the Thoracic Clinic of Guangdong Provincial People’s Hospital (both online and offline). The planned recruiting period is from Jan 1, 2021 to Sep 30, 2021, including a pilot study for the calculation of the sample size. Final analysis is expected before Sep 30, 2022, after 1-year follow-up of all the patients. The data in the pilot study will also be included in final analysis. During the follow-up period, patients who were advised to continue follow-up surveillance but underwent surgical resection will be enrolled into surgical group. The remaining patients who adhere to the surveillance plan will be automatically classified as follow-up group after 1-year follow-up (Figure 1). The psychological morbidity measures will be embedded within real-world services, using as a reference for shared decision-making. This real-world design aims to observe the interactive effect between patients’ psychological status and their spontaneous healthcare-seeking behaviors, and is different from the trial setting of previous studies.
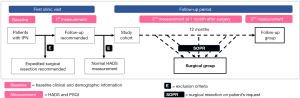
Study objectives
Primary objectives
To ascertain the benefit of surgery to the IPN patients with abnormal psychological states, and to establish a cutoff value of psychological morbidity measures as an optimal threshold for surgical intervention.
Secondary objectives
- To identify potential factors contributing to different healthcare-seeking behavior between the surgical and the follow-up groups;
- To evaluate the correlation among HADS, Pittsburgh Sleep Quality Index (PSQI) and indicator of healthcare-seeking behavior, and validate their feasibility to serve as anchors of psychological states;
- To evaluate the possibility of simplifying the psychological screening tool by examining the correlation between the comprehensive psychological morbidity measure (HADS) and single screening question (distress thermometer);
- To characterize the relationship between patients’ psychological distress and their individual characteristics (demographic features, disease-related features, personal behaviors or beliefs, etc.).
Exploratory objectives
To identify modifiable variables and explore the effectiveness of alternative methods or services (patient education or psychological counseling) to mitigate psychological burdens in IPN patients who seek unnecessary surgery.
Inclusion criteria
First-round enrollment: patients will be enrolled for baseline screening of psychological distress based on the following criteria:
- Aged 18 years and above;
- Diagnosed with solitary or multiple ≤3 cm IPN by high-resolution CT scan;
- Ability to understand and complete the questionnaires;
- Ability to give informed consent.
Second-round enrollment: After the initial psychological screening by HADS (HADS-A for anxiety subscale, HADS-D for depression subscale), the following criteria will be used to enroll patients into the formal study cohort:
- Abnormal or borderline psychological screening results (scores ≥8 in either subscale of HADS);
- Consent to participate in the follow-up surveillance.
Exclusion criteria
First-round exclusion: patients will be excluded from the baseline psychological screening if they demonstrate any of the following criteria:
- Known pathological diagnosis of pulmonary nodules by biopsy;
- Previous history of malignancy in any site of the body;
- Expedited surgical resection instead of follow-up surveillance recommended for the treatment of pulmonary nodules.
Second-round exclusion: patients will be further excluded from the final analysis if they have a normal psychological screening results (scores <8 in both of the HADS subscale).
Data collection and management
Upon the initiation of this study, the research protocol and data collection form were predefined and reviewed by a multidisciplinary expert panel which included surgeons, public health specialists, statistician, and patient representatives. The baseline data of interest include age, gender, education level, smoking status, family history of malignancy or lung cancer, risk factor exposure, symptomatic complaints, reasons for CT workup, number and size of pulmonary nodules, dynamic change of PN during follow-up, impression and treatment recommendation for PNs from doctors, patient treatment preference, frequency of seeking disease-related information (DRI), self-reported knowledge to PNs, as well as anxiety level assessed by one-question distress thermometer. A web-based questionnaire in Chinese will be delivered to all online and offline PN patients who visit the thoracic clinic during the study period. These patients come from different regions of China and surgical treatment offered will not only be limited to our institution, thus showing wide geographical representation and ensuring generalizability of the study outcome. The patients will then be assessed for eligibility through reviewing of the abovementioned baseline information.
HADS, a well-validated instrument comprising of two subscales (each with a score ranged from 0 to 21), will then be used to measure the level of anxiety (HADS-A) or depression (HADS-D) after the first-round of exclusion of the patients. Subscale scores of 0–7, 8–10 or ≥11 are defined as normal, borderline or abnormal psychological status, respectively (19). On the other hand, PSQI, a self-rated questionnaire which assesses sleep quality and disturbances over 1 month, will be collected to serve as an indicator for psychological burden. During follow-up period, patients in the surgical group will receive a second HADS and PSQI measurement approximately 1 month after surgery. The 1-month interval is a washout period for patients to get through the emotional perturbation brought about by surgery and pathological diagnosis. Otherwise, patients will be automatically categorized to follow-up group and will receive second assessment at the end of the 1-year follow-up.
Web-based questionnaire used in this study is powered by Wen Juan Xing (WJX, www.wjx.cn), the largest online platform for design and delivery of surveys in China. Patients who do not have or cannot access internet services will complete their questionnaires on the researchers’ pad, by paper or orally. All the enrolled patients will be educated to update their status once they undergo surgery. They will also be approached by researchers every 1 to 2 months to prevent any data dropout.
Study outcomes
Primary outcomes of interest will include: levels of anxiety (HADS-A1) or depression (HADS-D1) at baseline, which will be measured by the initial HADS scores (HADS1); levels of anxiety (HADS-A2) or depression (HADS-D2) at follow-up, which will be measured by the second HADS scores (HADS2); dynamic change of psychological burden (ΔHADS), which will be calculated by HADS2 minus HADS1. Other outcomes of interest will include: the level of distress measured by distress thermometer (included in baseline information); correlation between distress thermometer and HADS; correlation between HADS and PSQI; proportion of patients with abnormal or borderline psychological state that would improve to normal state after follow-up or surgical resection; effect sizes of various patient characteristics (demographic, disease profile, behavior or belief) that would contribute to the psychological burden.
Pilot study and sample size calculation
A pilot study involving 113 patients between Jan 1st, 2021 to Jan 31st, 2021 was conducted to provide reference for sample size calculation. The median follow-up period of the pilot cohort was 6 months as shown in the flowchart detailing the study design and patient selection (Figure 2). The baseline information of 70 patients after first-round selection is summarized in Table 1. Out of the total patients, 28.6% (20/70) had an abnormal or borderline abnormal HADS measurement at baseline. After a median follow-up of 6 months, 25.0% (5/20) of the patients received surgery while the remaining adhered to their follow-up plan. Variations in the HADS measurements of the surgical group (n=5) and follow-up group (n=15) are shown in Table 2. Data from the pilot study demonstrated that the ratio of patients in the surgical and follow-up groups of the planned study is expected to be 1:3. The differences of anxiety and depression measurements between baseline and follow-up and their corresponding standard deviations (SD) were −6.00±6.08 and −5.67±2.52, respectively, in the surgical group. In contrast, the differences of anxiety and depression measurements between the baseline and follow-up were −4.07±2.91 and −3.67±3.71, respectively, in the follow-up group. The sample size calculation was based on the independent sample Student’s t-test, using a two-tailed alpha level of 0.05 and a beta error probability of 0.2 (80% power). The required sample size for the final analysis is about 85 patients in the surgical group and 254 patients in the follow-up group. Considering a 10% attrition rate according to our previous experience, we will need 94 (85/0.9) and 282 (254/0.9) patients, respectively, in the two groups. Given that approximately 30% of participants had abnormal or borderline abnormal psychological state, at least 1,253 [(94+282)/0.3] IPN patients should be enrolled for our baseline psychological screening.
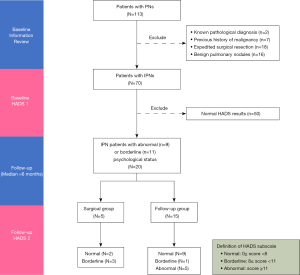
Table 1
| Variables | Patients with IPN, n (%) |
|---|---|
| Age (years) | |
| <60 | 64 (91.4) |
| ≥60 | 6 (8.6) |
| Gender | |
| Male | 24 (34.3) |
| Female | 46 (65.7) |
| Smoking status† | |
| Current | 2 (2.9) |
| Ever | 12 (17.7) |
| Never | 54 (79.4) |
| Family Hx of malignancy† | |
| No | 33 (48.5) |
| Yes | 35 (51.5) |
| Family Hx of lung cancer† | |
| No | 53 (77.9) |
| Yes | 15 (22.1) |
| Risk factor exposure† | |
| No | 27 (39.7) |
| Yes | 41 (60.3) |
| Subjective symptoms | |
| No | 43 (61.4) |
| Yes | 27 (38.6) |
| Reasons of CT workup† | |
| Physical discomfort | 13 (19.1) |
| Health check-up | 25 (36.8) |
| Follow-up exam for PN | 30 (44.1) |
| Number of pulmonary nodules | |
| Solitary | 42 (60.0) |
| Multiple | 28 (40.0) |
| Size of pulmonary nodule | |
| ≤10 mm | 59 (84.3) |
| 11 to 20 mm | 10 (14.3) |
| 21 to 30 mm | 1 (1.4) |
| Patient treatment preference† | |
| Surgery | 27 (39.7) |
| Medication | 4 (5.9) |
| Follow-up | 35 (51.5) |
| Others | 2 (2.9) |
| Frequency of exposure to related PHE | |
| Never | 2 (2.9) |
| Weekly | 37 (52.9) |
| Monthly | 18 (25.7) |
| Yearly | 13 (18.6) |
| Pathological diagnosis after resection | |
| Malignant | 13 (86.7) |
| Benign | 2 (13.3) |
| HADS-A at clinic visit (HADS-A1) | |
| Normal | 50 (71.4) |
| Borderline | 11 (15.7) |
| Abnormal | 9 (12.9) |
| HADS-A at follow-up (HADS-A2) | |
| Normal | 55 (78.6) |
| Borderline | 9 (12.9) |
| Abnormal | 6 (8.6) |
| HADS-D at clinic visit (HADS-D1) | |
| Normal | 58 (82.9) |
| Borderline | 7 (10.0) |
| Abnormal | 5 (7.1) |
| HADS-D at follow-up (HADS-D2) | |
| Normal | 60 (85.7) |
| Borderline | 4 (5.7) |
| Abnormal | 6 (8.6) |
†, data was missing in two patients. CT, computed tomography; IPN, indeterminate pulmonary nodules; Hx, history; PHE, patient health education; PN, pulmonary nodules; HADS, Hospital Anxiety and Depression Scale; -A or -D, subscales of anxiety or depression.
Table 2
| HADS scores (mean ± SD) | Surgical group (n=5) | Follow-up (n=15) |
|---|---|---|
| HADS-A1 | 11.8±3.63 | 11.93±4.33 |
| HADS-A2 | 5.8±4.44 | 7.87±5.32 |
| HADS-D1 | 10.33±0.58 | 11.33±3.87 |
| HADS-D2 | 4.67±2.52 | 7.67±5.20 |
| Change of HADS-A (A2-A1) | −6.00±6.08 | −4.07±2.91 |
| Change of HADS-D (D2-D1) | −5.67±2.52 | −3.67±3.71 |
HADS, Hospital Anxiety and Depression Scale; -A or -D, subscales of anxiety or depression; SD, standard deviation.
Statistical analysis
Statistical analyses will be performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA). In this study, categorical variables will be reported as frequency and percentage, while continuous variables will be presented by mean and SD, or median and interquartile ranges (IQR). To compare the baseline characteristics between groups, Chi-square test or nonparametric test will be used based on the variable feature. To compare the original or derived HADS scores between the groups, independent-samples t-test or analysis of variance (ANOVA) will be used as appropriate. Optimal cut-point for surgical alert threshold will be generated using the receiver operating characteristics (ROC) analysis. For the secondary objectives, correlation between HADS and distress thermometer will be assessed by Pearson correlation coefficient analysis. Cohen’s guidelines for the interpretation of correlation coefficients will be used with a correlation of 0.10 being small, a correlation of 0.30 being moderate, while 0.50 and above will indicate a strong correlation. Effect sizes of various patient characteristics (demographics, disease profile, behaviors or beliefs) that contribute to psychological burden will be analyzed using general linear regression. In addition, subgroup analysis will be performed to investigate the influence of exposure to related patient health education, treatment recommendation from doctors, patient treatment preference and duration of follow-up. Multivariate analysis or propensity score matching analysis will be employed to adjust for confounding factors where necessary. A two-sided P value of <0.05 will be considered statistically significant in all tests.
Ethical approval and trial registration
This study was approved by the medical ethics committee of Guangdong Provincial People’s Hospital (KY-Q-2021-005-03) and will be conducted following the guideline of the Declaration of Helsinki (as revised in 2013). Informed consents for data collection will be obtained from all participants at their first visit to the clinic. This study was registered in ClinicalTrial.gov (registration No. NCT04857333) with an official title of “Dynamic Evolution of Pulmonary Nodules and Influence Factors of Its Clinical Decision-making: A Prospective Cohort Study (DEPICT)”.
Safety and confidentiality
Enrollment in this observational study is not associated with risks in any aspect, and no financial compensation will be rewarded to the participants. Identifiable personal information will be replaced by codes and kept confidential throughout the data analysis process and public reports. Researchers, research authorities, and ethical committees will be allowed to access the original data for the sole purpose of research supervision.
Discussion
With the popularization of CT scan in the annual physical checkup, China has experienced a dramatically increased prevalence of PN in recent years. The psychological burden that results from IPN has been reported in many population-based studies (9,10,17,18), and the distress-related impairment in HRQoL might exceed the nodule-specific detriment. Current recommendations to clinical practice have taken the psychological aspect into consideration, which has more reliance on the patient-centered communication and shared decision-making (14). Nonetheless, there is lack of appropriate psychological screening tools in the clinic setting. Subjective judgement by physicians or surgeons in a few minutes of communication might overestimate or underestimate the extent of psychological issues. So far, there is no established criterion to guide the process of patient selection, and to ensure an evidence-based management. This real-world prospective study is designed based on the unmet clinical needs, and aims to contribute towards an adoption a strategy that would improve the quality of care for IPN patients.
Although some of the previous studies have demonstrated the dynamic change of psychological burden in patients with indeterminate pulmonary nodules (9,10,17,18), none of them examined the influence of surgical resection or pathological diagnosis. Other large-scale studies only compared the psychological changes between the CT group and control group, and showed no significant variation (11,20-22). Our pilot study demonstrated that only about 30% of patients were found to have abnormal or borderline abnormal psychological burden. Thus, the psychological change in these patients may be covered or diluted by the other 70% normal samples in previous studies. Therefore, to test the hypothesis of the planned study, it is necessary to exclude the psychologically normal IPN patients from final analysis.
The strength of this study will be bolstered by its prospective design and real-world clinical setting, which will reflect the clinical reality and provide valid evidence for shared decision-making. In addition, a well-conducted pilot study helped to establish a standard operation procedure for data collection as well as calculation of the sample size. This study will be powered by the appropriately determined sample size and the rigorous inclusion and exclusion criteria. Moreover, patients recruited from all over the country might receive surgery at their local hospitals, which will ensure the geographical representation and minimize potential selection bias. Nevertheless, our study is limited by the fact that qualitative interview by psychologist, which is costly and time-consuming, will not be used to validate the HADS results of the IPN patients, though HADS has been proofed as a valid and reliable measurement in many previous studies (15,19,23,24).
Conclusions
This study is a prospective cohort study in real-world setting to examine the psychological changes related to different healthcare-seeking behaviors among IPN patients. The results are expected to answer whether psychological distress could be listed as surgical indication for IPN, and to establish an evidence-based distress threshold for necessary surgical interventions.
Acknowledgments
The authors thank the Freescience Editorial Team for their proofreading and editing for grammar and language clarity.
Funding: This study was funded by the 2020 Guangdong Provincial Special Project for Popularization of Science and Technology Innovation (grant number: 2020A1414070007).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1423/coif). All authors report funding from the 2020 Guangdong Provincial Special Project for Popularization of Science and Technology Innovation (grant number: 2020A1414070007). The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Zheng C, Huang BZ, Agazaryan AA, et al. Natural Language Processing to Identify Pulmonary Nodules and Extract Nodule Characteristics From Radiology Reports. Chest 2021;160:1902-14. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Wu GX, Raz DJ, Brown L, et al. Psychological Burden Associated With Lung Cancer Screening: A Systematic Review. Clin Lung Cancer 2016;17:315-24. [Crossref] [PubMed]
- Bauml JM, Troxel A, Epperson CN, et al. Scan-associated distress in lung cancer: Quantifying the impact of "scanxiety". Lung Cancer 2016;100:110-3. [Crossref] [PubMed]
- Slatore CG, Wiener RS, Golden SE, et al. Longitudinal Assessment of Distress among Veterans with Incidental Pulmonary Nodules. Ann Am Thorac Soc 2016;13:1983-91. [Crossref] [PubMed]
- Slatore CG, Sullivan DR, Pappas M, et al. Patient-centered outcomes among lung cancer screening recipients with computed tomography: a systematic review. J Thorac Oncol 2014;9:927-34. [Crossref] [PubMed]
- Quaife SL, Janes SM, Brain KE. The person behind the nodule: a narrative review of the psychological impact of lung cancer screening. Transl Lung Cancer Res 2021;10:2427-40. [Crossref] [PubMed]
- Byrne MM, Weissfeld J, Roberts MS. Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening. Med Decis Making 2008;28:917-25. [Crossref] [PubMed]
- van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON). Br J Cancer 2010;102:27-34. [Crossref] [PubMed]
- van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Long-term effects of lung cancer computed tomography screening on health-related quality of life: the NELSON trial. Eur Respir J 2011;38:154-61. [Crossref] [PubMed]
- Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology 2009;251:175-84. [Crossref] [PubMed]
- McCunney RJ, Li J. Radiation Risks in Lung Cancer Screening Programs. Chest 2014;145:618-24. [Crossref]
- Jiang G, Chen C, Zhu Y, et al. Shanghai Pulmonary Hospital Experts Consensus on the Management of Ground-Glass Nodules Suspected as Lung Adenocarcinoma (Version 1). Zhongguo Fei Ai Za Zhi 2018;21:147-59. [PubMed]
- Annunziata MA, Muzzatti B, Bidoli E, et al. Hospital Anxiety and Depression Scale (HADS) accuracy in cancer patients. Support Care Cancer 2020;28:3921-6. [Crossref] [PubMed]
- Annunziata MA, Muzzatti B, Altoè G. Defining hospital anxiety and depression scale (HADS) structure by confirmatory factor analysis: a contribution to validation for oncological settings. Ann Oncol 2011;22:2330-3. [Crossref] [PubMed]
- Brain K, Lifford KJ, Carter B, et al. Long-term psychosocial outcomes of low-dose CT screening: results of the UK Lung Cancer Screening randomised controlled trial. Thorax 2016;71:996-1005. [Crossref] [PubMed]
- Kummer S, Waller J, Ruparel M, et al. Psychological outcomes of low-dose CT lung cancer screening in a multisite demonstration screening pilot: the Lung Screen Uptake Trial (LSUT). Thorax 2020;75:1065-73. [PubMed]
- Li Q, Lin Y, Hu C, et al. The Chinese version of hospital anxiety and depression scale: Psychometric properties in Chinese cancer patients and their family caregivers. Eur J Oncol Nurs 2016;25:16-23. [Crossref] [PubMed]
- Kaerlev L, Iachina M, Pedersen JH, et al. CT-Screening for lung cancer does not increase the use of anxiolytic or antidepressant medication. BMC Cancer 2012;12:188. [Crossref] [PubMed]
- van den Bergh KA, Essink-Bot ML, Bunge EM, et al. Impact of computed tomography screening for lung cancer on participants in a randomized controlled trial (NELSON trial). Cancer 2008;113:396-404. [Crossref] [PubMed]
- Aggestrup LM, Hestbech MS, Siersma V, et al. Psychosocial consequences of allocation to lung cancer screening: a randomised controlled trial. BMJ Open 2012;2:e000663. [Crossref] [PubMed]
- Esser P, Hartung TJ, Friedrich M, et al. The Generalized Anxiety Disorder Screener (GAD-7) and the anxiety module of the Hospital and Depression Scale (HADS-A) as screening tools for generalized anxiety disorder among cancer patients. Psychooncology 2018;27:1509-16. [Crossref] [PubMed]
- Lambert SD, Pallant JF, Boyes AW, et al. A Rasch analysis of the Hospital Anxiety and Depression Scale (HADS) among cancer survivors. Psychol Assess 2013;25:379-90. [Crossref] [PubMed]


