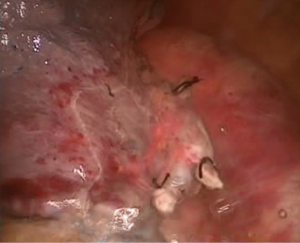
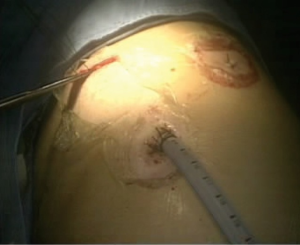
Complete video-assisted thoracoscopic lobectomy of the left lower lobe and lung lymph node dissection
Introduction
Lung cancer is the most common malignancy worldwide. In the past 50 years, the incidence and mortality of lung cancer have exhibited an increasing trend. The most recent statistical data obtained by the American Cancer Society (ACS) in 2014 indicate that in both men and women, the incidence of lung cancer is second highest among all malignant tumors, and lung cancer is the leading cause of cancer mortality in both men and women. Lung-cancer-associated deaths represent more than 25% of all cancer deaths, and lung cancer has become the deadliest cancer for both men and women (1). With the worsening of air pollution in China and the increase in the PM2.5 index, lung cancer has become the most common malignant tumor affecting urban residents, and its incidence and mortality rank first among all types of tumors (2). Disease onset primarily occurs at 40 years or older, and the peak onset age is between 60 and 79 years old. The prevalence ratio of men to women is 2.3:1. Surgery is the most effective method used to treat lung cancer.
The initial development of minimally invasive thoracic surgery, represented by video-assisted thoracoscopic surgery (VATS), occurred in the 1990s (3,4), and VATS was quickly applied to the surgical treatment of lung cancer. After 2004, reports on the minimally invasive treatment of lung cancer increased dramatically. Beginning in 2006, the VATS lobectomy was included in the guidelines of the National Comprehensive Cancer Network (NCCN) and the American College of Chest Physicians (ACCP). The VATS lobectomy has become one of the standard surgical methods for lung cancer treatment and a basic skill that a new generation of thoracic surgeons must know. Compared with a traditional thoracotomy, a VATS lobectomy has the advantages of producing less trauma, less bleeding, a faster recovery, less scarring, and a shorter hospitalization period.
Video summary
The patient was a 67-year-old man. A physical examination revealed a nodule in the left lower lobe that had been present for 7 years. No clinical symptoms or positive signs were noted. According to the chest computed tomography (CT) report, a basal segment nodule 1.8 cm × 3.1 cm in size was observed in left lower lobe; the density of the nodule was uniform, and its edges were irregular with lobulation. Enlarged lymph nodes were not observed in the hilum and mediastinum (Figure 1A,B). Further study of the lobe characteristics was needed, and a diagnosis of lung cancer was not excluded. Brain magnetic resonance (MR) images, a bone scan and abdominal ultrasound did not reveal distant tumor metastasis. Due to the surgical indications, we opted to perform either a complete thoracoscopic lung wedge resection, intraoperative freezing of pathological sections or a lobectomy. After the thoracoscopic examination, the left lower lobe lesion was determined to be located in the basal segment of the left lower lobe close to the left inferior pulmonary vein, and we were unable to perform a wedge resection of the lesion. Moreover, the appearance was highly suggestive of cancer; therefore, a direct lobectomy and lymphadenectomy of the hilum and mediastinum were performed. The basilar trunk and dorsal segment of the left pulmonary artery, left inferior pulmonary vein, and lower lobe bronchus were sequentially processed. Specimens were obtained, and each group of lymph nodes was cleaned. The frozen pathology report was consistent with adenocarcinoma.
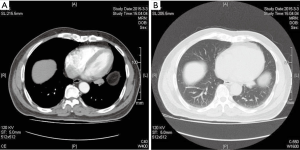
The CT images are shown in Figure 1.
Video description
Choice of incision
An observation port was created on the right midaxillary line in the 7th intercostal space (perpendicular intersection of the horizontal lines of the anterior superior iliac spine and xiphoid process).
The main incision was located on the right anterior axillary line in the 5th intercostal space.
The secondary incision was located on the right posterior axillary line in the 7th intercostal space (the same intercostal space as the observation port).
Surgical instruments used
(I) Blunt end electrocoagulation hook. (II) Endoscopic blunt end multi-hole aspirator. (III) Toothed curved sponge forceps and toothless curved sponge forceps. (IV) Pedicle clamp. (V) Johnson-Johnson ECHELON endoscopic linear stapler (Ethicon Endo-Surgery, Inc., a Johnson-Johnson company, USA) (equipped with a blue, white, and green cartridge). (VI) HARMONIC (ACE) Scalpel.
Manipulation techniques
The operation was undertaken under general anesthesia, single-lumen endotracheal intubation, left lung blockage and one-lung ventilation. One 1.0-cm observation port was made on the left midaxillary line in the seventh intercostal space; the main incision (~3 cm) was located on the left anterior axillary line in the 5th intercostal space. The secondary incision, which was generated by puncture using a 5-mm endoscopic puncture needle, was located on the left posterior axillary line in the 7th intercostal space. The instruments used included the following: a blunt-end electrocoagulation hook, a endoscopic blunt-end multi-hole aspirator, toothed curved sponge forceps, toothless curved sponge forceps, a pedicle clamp, a Johnson-Johnson ECHELON endoscopic linear stapler (equipped with a blue, white, and green cartridge), and a HARMONIC (ACE) Scalpel. These simple instruments effectively meet the requirements of the operation.
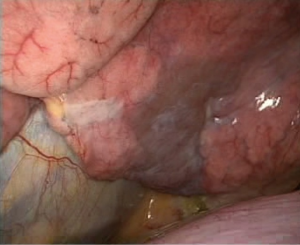
After a thoracoscopic examination, the left lower lobe lesion was determined to be located in the basal segment of the left lower lobe close to left inferior pulmonary vein, and we were unable to perform a wedge resection of the lesion. Moreover, the appearance was highly suggestive of cancer; therefore, a direct lobectomy was performed (Figure 2).
The detailed operation procedures were as follows:
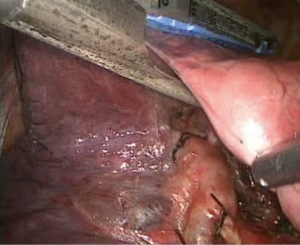
- First, the lower lung was lifted upward to expose the lower lung ligament, and the HARMONIC (ACE) Scalpel was used to separate the lower lung ligament until the inferior pulmonary vein was reached (Figure 3); simultaneously, a lymphadenectomy was performed for the lower lung ligament lymph nodes;
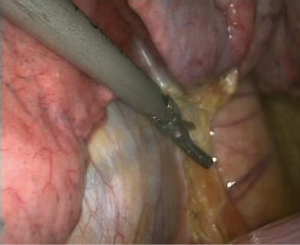
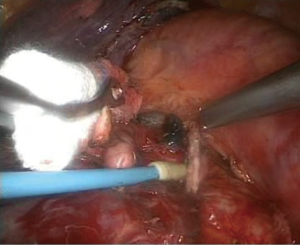
- Processing of the basilar trunk and dorsal segment of the left pulmonary artery. An electrocoagulation hook was used to separate the oblique fissure along the gap of the lung fissure from the lingula of the left upper lobe until the left main trunk and basilar trunk of the left pulmonary artery were exposed. The electrocoagulation hook was used for separation by electric coagulation of the vascular sheath and blunt dissection. We attempted to maximally push the loose connective tissue in the sheath to the side so that each blood vessel was separated and “skeletonized”. No. 4 thread was used to ligate the proximal end, and a HARMONIC (ACE) Scalpel was used to remove the distal end (Figures 4,5);
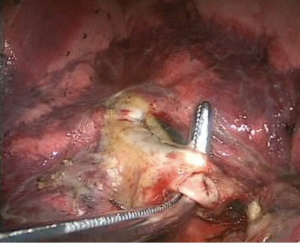
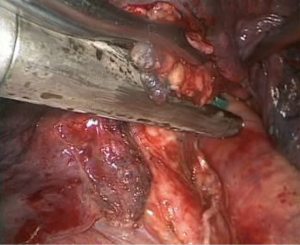
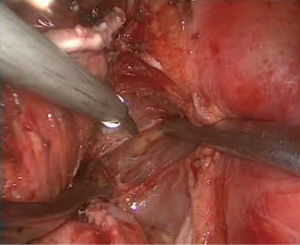
- Complete separation of the oblique fissure. A HARMONIC (ACE) Scalpel and an electrocoagulation hook were used alternatively from bottom to top to separate the posterior mediastinal pleura until the fissure was reached. Simultaneously, the main trunk of the left pulmonary artery was exposed, which formed a tunnel with the separated fissure at the lingula, and an ECHELON stapler was used to cut and suture the completely separated fissures along the tunnel (Figure 6);
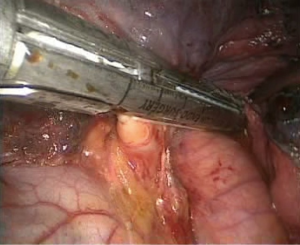
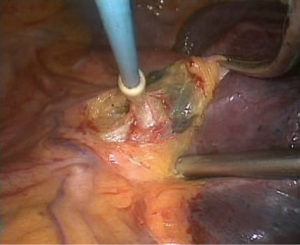
- Processing of the inferior pulmonary vein. An electrocoagulation hook was used to open the vascular sheath of the inferior pulmonary vein; the surrounding tissues of the vein were separated, and the blood vessels in the vascular sheath were separated. A clamp and a No. 7 thread were used to lift the right inferior luminary vein to expose the vascular space and allow the ECHELON to pass through (Figure 7), and the blood vessels were cut;
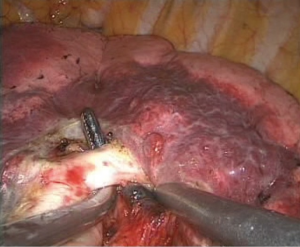
- Processing of the lower lobe bronchus. The lung tissue was pulled up and forward, and the lower lobe bronchus was exposed. Electrocoagulation was achieved with the electrocoagulation hook, and the aspirator was used to bluntly separate the lymph nodes around the bronchus. A vascular clamp was passed through the lower lobe bronchus, and sponge forceps were used to lift the left lower lobe bronchus to expose the space behind the bronchus. After passing the ECHELON through the bronchus and closing it (Figure 8), the anesthetist suctioned the phlegm and expanded the lung. Subsequently, the lower lobe bronchus was cut, and a complete resection of left lower lobe was performed;
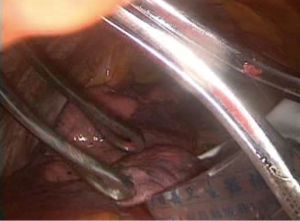
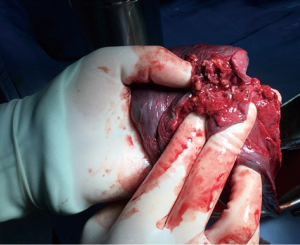
- Specimen collection. We used homemade specimen bags (disposable drainage bag) to contain the specimens, which were removed through the main incision (Figures 9,10), thereby avoiding seeding metastases in the incision of the tumor;
- Lymphadenectomy of the hilum and mediastinum. Cauterization, aspiration and other instruments were used to resect the 10th, 7th, 8th, 9th, 5th, 6th and 4L group of lymph nodes (Figures 11-13);
- Completion of hemostasis and wound rinsing. After the remainder of the lung was tested with water and showed no leakage, a No. 30 drainage tube was placed through the observation port (Figures 14,15). Before the chest was closed, we used a scope to confirm that the remaining lung was well expanded, and the incision was closed layer by layer.
Comments and discussion
The surgical procedures illustrated in the video represent a complete thoracoscopic left lower lobectomy and lymphadenectomy of the hilum and mediastinum. This procedure reflects the essence of the three concepts of “minimally invasive radical surgery for lung cancer” suggested by Professor Jianxing He at the First Affiliated Hospital of Guangzhou Medical University (5). These concepts aim to obtain consistent surgical tumor outcomes. First, the damage to various organ functions secondary to the surgical operation should be minimal. Second, the incision should be significantly smaller than a traditional incision. The number of incisions required for a thoracoscopy is less than four, and the secondary incision length should be shorter than 4 cm. Third, more than three areas of the mediastinum should be cleaned, and the number of lymph nodes should range from 11 to 17, which includes seven groups of lymph nodes. In this video, the surgeon is highly skillful through the entire operation, and the operation is performed smoothly, without delays. The surgeon has a clear understanding of the anatomy, and through pulling, pushing and other techniques, the surgical field can be exposed completely. Flexible and ingenious utilization of the electrocoagulation hook, HARMONIC (ACE) Scalpel, ECHELON stapler and other instruments allow the accomplishment of the operation. With respect to the selection of the incision, this procedure uses the left midaxillary line in the 7th intercostal space to make a 1.0 cm observation port; the main incision is located on the left anterior midaxillary line in the 5th intercostal space, and the length is approximately 3 cm. The secondary incision is located on the posterior midaxillary line in the 7th intercostal space, which is made using a 5-mm endoscopic puncture needle; an assistant can use this opening to manipulate the aspirator and HARMONIC (ACE) Scalpel. This opening is located in the same intercostal space as the observation port so that the probability of intercostal nerve injury is reduced, and postoperative symptoms can be improved. When managing the pulmonary artery and pulmonary vein, the electrocoagulation hook and aspirator work together to complete the separation and dissection within the vascular sheath, maximally pushing away the loose connective tissues in the sheath and eventually achieving “skeletonization” through blood vessel dissection. These tools can also dissect sufficient blood vessels and expose adequate vascular space to avoid pulling, tearing and bleeding while cutting. When using the ECHELON to clip and cut the left lower pulmonary vein, the surgeons pull the trigger quickly. If the procedure can be performed after the blood vessel or fissure is clamped for 15 s, bleeding and leakage from the stump may be more efficiently avoided, thereby avoiding the need to repeat the operation. When separating the fissure, a tunnel-type fissure separation method is used. The HARMONIC (ACE) Scalpel and electrocoagulation hook are used in a direction from the bottom to the top to separate the posterior mediastinal pleura alternatively until the fissure is reached, simultaneously exposing the main trunk of the left pulmonary artery and forming a tunnel with the separated fissure at the lingula. The ECHELON is used to cut and suture the completely separate fissures along the tunnel, and the basal anatomy of the left lower lobe is exposed more clearly. The overall operation sequence is oblique fissure–pulmonary artery–pulmonary vein–bronchi. However, the surgeon should not be restricted to a particular method; specifically, the surgeon does not need to simply follow the unidirectional lobectomy of the West China Hospital or Wang’s procedure of Peking University Hospital. The operation should be performed according to the surgeon’s habits, the development of the fissure and variation in the blood vessels. Lymphadenectomy of the hilum and mediastinum is a necessary step for the radical resection of malignant lung tumors; for the 10th and 11th groups of lymph nodes, the safest and most effective method is to open a vascular sheath for cleaning. The blood supply of the mediastinal lymph nodes arises primarily from the bronchial artery. The lymph node bronchial artery supply side must first be separated while the bronchial artery is cut to avoid bleeding that obscures the surgical field during lymph node cleaning. The cleaning of the 5th and 6th group of lymph nodes is the same as that of the 2nd and the 4th groups of the right mediastinum. We recommend an overall resection to avoid lymph node rupture, bleeding and incomplete resection. In this area, attention must be paid so that the recurrent laryngeal nerve is not injured. In the present video, there is inadequate exposure of this nerve. If the assistant holding the endoscope can achieve better exposure by pushing the thoracoscope approximately 5 cm away from the operation center to achieve the optimal local magnification effect, the surgeon can identify the nerve more clearly.
Complete thoracoscopic lobectomy has been widely promoted in China and has become one of the most popular techniques, attracting significant attention in the thoracic surgery field. Compared with a traditional thoracotomy, VATS radical surgery for lung cancer causes less pain, requires less chest tube drainage, is associated with shorter hospitalization time, and leads to faster postoperative recovery. To successfully perform this operation, the following five conditions are needed: (I) clearer thoracoscopic equipment; (II) good surgical field exposure and the help of a good assistant holding the thoracoscope; (III) skillful microscopic vascular anatomic separation techniques; (IV) the ability to carefully place blood vessels and the bronchus into a stapler; and (V) the mediastinal lymphadenectomy technique (6).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Consents: The authors agree to the “Copyright Transfer Agreement”, and confirm that the patient who appears in this video has signed a consent form.
References
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252-71. [PubMed]
- Wang G, Zeng S, He P, et al. Investigation and Analysis of Incidence and Mortality of Lung Cancer in Wenjiang District, Chengdu City in 2008–2010. Practical Preventive Medicine 2013;20:1314-6.
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Kirby TJ, Rice TW. Thoracoscopic lobectomy. Ann Thorac Surg 1993;56:784-6. [PubMed]
- He J, Shao W, Cao C, et al. Long-term outcome and cost-effectiveness of complete versus assisted video-assisted thoracic surgery for non-small cell lung cancer. J Surg Oncol 2011;104:162-8. [PubMed]
- Li Y, Wang J, Sui XZ, et al. Operative technique optimization in completely thoracoscopic lobectomy: Peking University experience. Chin J Thorac Cardiovasc Surg 2010;26:300-6.