
Clinical diagnostic algorithm in defining tuberculous unilateral pleural effusion in high tuberculosis burden areas short of diagnostic tools
Introduction
Abnormal accumulation of pleural effusion (PE) in the pleural cavity, a common condition in clinics, can be caused by various diseases, with an estimated 1.5 million new cases each year (1). Reportedly, PE development involves with more than 50 etiologies (2), but is usually categorized into transudative and exudative effusions based on biochemical characteristics, which can be easily distinguished using Light’s criteria (1). However, the parameters commonly measured in PEs rarely contribute to the diagnosis of the underlying disease as evidenced in some studies where the exact cause was not determined in approximately 20% of PE cases (3). Because different causes are associated with different management procedures, diagnosing a precise cause of PE is important (4).
Etiologically, heart failure, pneumonia, neoplasm, and tuberculous pleurisy were found to be the leading causes of PE (5). In China and many developing countries, pleural tuberculosis (TB) has been estimated to account for 6.5–8.7% of all TB cases (6), and considering the high prevalence of TB, tuberculous PE (TPE) is a major cause of all PE cases (7). However, the differentiation of TPE from other exudative PEs, especially malignant PE (MPE), remains a clinical challenge due to the similar clinical and laboratory manifestations presented and occasional lack of pathological or etiological evidence (8). Also, the paucibacillary TB and the slow growth of mycobacteria in the conventional culture media delays the precise diagnosis of TPE (9). Hence, more invasive pleural procedures, such as closed pleural biopsy or thoracoscopy for histological analysis, are needed to determine the final diagnosis (10,11).
Given that microbiological and pathological diagnoses can rarely be achieved and, more importantly, many sophisticated detection methods were not available in many areas of high TB burden, biomarkers have been introduced to correctly diagnose TPE, including protein and molecular biomarkers (12). Among them, the adenosine deaminase (ADA) and interferon-gamma release assay (IGRA) are reported to be helpful in the diagnosis of TPE (13). However, each method has shortcomings and may be influenced by many external factors. To reduce the high prevalence of TB and improve early management of TB, the National Health and Family Planning Commission of China has released the criterion of mandatory standards of the health industry for the diagnosis of TB, which includes multiple testing methods and classifies the diagnosis into three levels. However, few studies have been published regarding the performance of this criterion in TPE diagnosis. In this paper, the effectiveness of this criterion in the diagnosis of TPE is reported in real world clinical settings, and we present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1532/rc).
Methods
Study population
This retrospective study was performed in the General Hospital of Western Theater Command from December 2016 to December 2017 and followed-up until February 2020. The inclusion criteria included the following: (I) >16 years of age; (II) present with unilateral PE based on chest ultrasound or CT examinations; (III) available clinical records and prognostic information. Patients who had PE in both chests, received anti-TB therapy or immunosuppressive therapy and were HIV seropositive were excluded. Finally, 924 eligible patients were enrolled in the present study.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of the General Hospital of Western Theater Command. As a retrospective study, informed consent was not sought from all enrolled patients.
Data collection and follow-up
Based on the primary diagnosis of PE, participants were classified into three groups: MPE (292 cases), TPE (239 cases), and other causes (393 cases). Patients in the TPE group included confirmed cases (36 cases) and clinically diagnosed cases (203 cases). The detailed classification and analysis procedures are presented in Figure 1. The baseline characteristics and test results were collected, including demographic data, effusion characteristics, levels of serum biomarkers, and blood tests. For patients of suspected tuberculous pleurisy, the drugs used for treatment and TB-related immunological results of tests were collected. Besides, the patients were followed up every 2–3 months with chest ultrasound or CT, and the results were also recorded. Treatment success was defined as the complete disappearance of PE in at least two consecutive follow-ups. Treatment failure was defined as the increase or recurrence of PE after treatment during follow-up.
Any newly diagnosed TPE patient, both confirmed and clinically diagnosed, was asked to receive World Health Organization (WHO) standard TB treatment regimen. Specifically, the entire treatment included at least a 2-month intensive phase of daily isoniazid, rifampin, pyrazinamide, and ethambutol followed by a 4-month continuous phase of daily isoniazid and rifampin.
Diagnostic criteria
Patients were preliminarily diagnosed according to the health industry standard criteria (WS 288-2017) by clinical doctor. This diagnostic algorithm includes three parts (14): confirmed cases, clinically diagnosed cases, and suspected cases. The confirmation of TB (Conf.) was defined as either one of the following: (I) presence of PE based on image examinations and pathological evidence from pleural biopsy (granulomatous inflammation with or without acid-fast bacilli (AFB) smear positive or PCR positive) from PE or pleura; (II) presence of PE and microbiological evidence from PE/sputum/lavage (Mycobacterium tuberculosis smear or culture). The clinical diagnosis of TPE (ClinD) was defined as the presence of PE, exudative PE according to Light's criteria, elevated level of ADA (>40 U/L) of PE, exclusion of other causes, and either of the following: (I) positive tuberculin purified protein derivative (PPD) test; (II) positive IGRA test; (III) positive M. tuberculosis antibody (TB-Ab) test. The suspicion of TPE (Susp.) was defined as the presence of PE and the exclusion of other causes. In this study, our diagnostic algorithm was limited to confirmed and clinical diagnoses.
The diagnosis of MPE was defined as the presence of PE and positive pleural fluid cytology and/or biopsy histology. Other causes were determined when diagnostic criteria were met precisely based on medical records. For two or more possible causes, the direct cause was selected.
Laboratory tests
In clinical settings, all patients in possible TPE groups and MPE groups underwent TB-related tests. Samples used for M. tuberculosis culture were obtained from pleural fluid/sputum/lavage. The AFB smears were performed with auramine-rhodamine fluorochrome. The mycobacterial cultures were processed using the standard N-acetyl-L-cysteine and sodium hydroxide method using the BACTEC MGIT 960 culture system (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). PCR was performed using an M. tuberculosis kit (Sansur Biotech, China) for the detection of M. tuberculosis-complex on pleural biopsy and effusion samples. The PPD test was performed using 5 IU of tuberculin pure protein derivative (Xiangrui Biological Products Co. Ltd, China). Serum TB-antibody was measured using an immunochromatographic test system (Alere Inc., China). The ADA level of pleural fluid was evaluated using an ADA assay kit (Maccura Biotech, China). The IGRA assay was performed using a commercial kit from Oxford Immunotec Ltd (UK). Medical thoracoscopy was performed using an EVIS EXERA LTF-160 pleura videoscope manufactured by Olympus (Tokyo, Japan). Around four to six pieces were collected in formalin for histopathological examination. All tests were performed following the respective manufacturer’s instructions.
Statistical analysis
Data were analyzed using R software (version 3.6.1, www.r-project.org) and GraphPad Prism (Version 6.01 San Diego, CA, USA). A comparison of variables between the two groups was performed using Mann-Whitney test and χ2 test for continuous variables (represented as median with 95% confidential interval, CI) and categorical variables (represented as number with percentage), respectively. A Venn diagram was depicted to compare the positive rate of different testing methods. Receiver operating curve (ROC) was depicted to compare the diagnostic efficacy and determine the area under ROC (AUC) and the best cut-off values. The 2×2 cross table was used to compare the sensitivity, specificity, and other indices. The golden standard was established as the final diagnosis after follow-up. Two-tailed P values <0.05 were considered statistically significant.
Results
Baseline patient characteristics
The baseline characteristics of three groups were listed in Table 1. First, the patients in the MPE group were significantly older than the patients in the TPE group (P<0.001). Also, the MPE group had a larger proportion of smoking patients than TPE group (P<0.001). Regarding PE characteristics, the level of pleural ADA in the TPE group was significantly higher than in the MPE group (47.2 vs. 10.1 U/mL, P<0.001). In addition, 238 PE cases in the TPE group were classified as exudate and 15 cases in the MPE group as transudate (P=0.006). Serum levels of all tumor biomarkers were significantly higher in the MPE group than in the TPE group (all P<0.01). For other blood tests, IGRA levels in the TPE group were nearly 80 times greater than in the MPE group (P<0.001). Consequently, these biomarkers could potentially help clinically distinguish TPE from MPE. Other causes of PE were listed in Table 1. Due to the heterogeneity of patients involved, data in these groups were not comparable.
Table 1
| Clinical characteristics | Possible tuberculous pleural effusion (N=239) | Malignant pleural effusion (N=292) | P | Others (N=393) |
|---|---|---|---|---|
| Age, years | 41.0 (26.0–60.0) | 63.0 (52.5–71.8) | <0.001 | 62.0 (48.0–71.0) |
| Gender, female | 69 (28.4%) | 108 (39.6%) | 0.720 | 132 (33.6%) |
| Length of stay, days | 15.4±8.5 | 18.8±15.7 | 0.097 | 15.4±13.8 |
| Location | 0.883 | |||
| Left | 95 | 105 | 150 | |
| Right | 148 | 168 | 243 | |
| Smoking history | 82 | 196 | <0.001 | 112 |
| PE characteristics | ||||
| Positive rivalta test | 181 | 206 | 0.181 | 45 |
| LDH (IU/mL) | 376.0 (250.2–587.0) | 333.4 (201.9–591.0) | 0.120 | 190.2 (63.0–503.1) |
| ADA (U/mL) | 47.2 (37.4–61.1) | 10.1 (8.0–14.0) | <0.001 | 9.7 (3.6–17.5) |
| Protein (g/l) | 48.9 (45.3–53.0) | 43.8 (36.7–47.8) | <0.001 | 29.8 (16.4–43.3) |
| Glu (mmol/L) | 5.3 (4.3–6.2) | 6.2 (5.0–7.6) | <0.001 | 7.1 (4.7–9.0) |
| Bloody effusion | 24 | 39 | 0.240 | 28 |
| Clotting | 168 | 185 | 0.092 | 56 |
| Exudate (light criteria) | 238 | 277 | 0.006 | |
| Tumor biomarkers | ||||
| CEA (U/mL) | 1.3 (0.8–2.1) | 10.7 (2.8–73.0) | <0.001 | 2.2 (1.4–3.5) |
| CYFRA21-1 (ng/mL) | 1.0 (0.7–1.4) | 6.4 (2.3–18.8) | <0.001 | 1.9 (1.1–3.0) |
| NSE (ng/mL) | 10.3 (5.1–12.5) | 13.0 (8.1–20.1) | <0.001 | 10.4 (6.1–13.5) |
| CA199 (U/mL) | 5.4 (3.0–9.4) | 12.9 (6.1–42.4) | <0.001 | 11.7 (6.2–25.6) |
| CA125 (U/mL) | 114.9 (59.2–202.6) | 136.8 (59.1–312.6) | 0.006 | 84.4 (31.4–237.3) |
| CA153 (U/mL) | 7.4 (5.6–11.7) | 16.1 (9.1–33.5) | <0.001 | 9.4 (6.1–18.6) |
| Blood tests | ||||
| AST (IU/L) | 19.7 (15.9–26.7) | 24.6 (17.6–33.2) | <0.001 | 28.3 (20.4–44.5) |
| ALT (IU/L) | 18.3 (11.1–31.2) | 20.5 (13.8–33.5) | 0.027 | 26.8 (15.6–45.4) |
| BNP (pg/mL) | 27.7 (12.8–59.8) | 34.0 (13.8–67.4) | 0.079 | 92.5 (34.6–304.3) |
| TK1 (pmol/L) | 2.8 (1.7–5.3) | 1.8 (1.0–3.4) | <0.001 | 2.0 (0.9–5.3) |
| IGRA (pg/mL) | 81.0 (30.2–232.2) | 1.4 (0.8–10.0) | <0.001 | 1.6 (0.7–16.5) |
PE, pleural effusion; LDH, lactate dehydrogenase; ADA, adenosine deaminase; Glu, serum glucose; CEA, carcinoembryonic antigen; CYFRA21-1, the fragment of cytokeratin 19; NSE, neuron-specific enolase; CA, carbohydrate antigen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BNP, brain natriuretic peptide; TK1, thymidine kinase 1; IGRA, interferon gamma release assay.
Empirical anti-TB treatment and follow-up
In addition to the confirmed TPE cases, 203 clinically diagnosed TPE cases were subjected to anti-TB procedure. 187 cases with at least one follow-up after receiving anti-TB treatment were included in the final analysis. The detailed prognosis and follow-up results are presented in Figure 2. Specifically, the follow-up results were separated into three parts: 1–3, 3–6, and 6–12 months after anti-TB treatment. Patients with confirmed and clinically diagnosed TPE received thorough closed thoracic drainage before leaving the hospital. After 1–3 months of standard anti-TB treatment, image examinations showed no signs of PE in most patients (152 cases). Furthermore, recurrence occurred in some patients and others recovered. Signs of PE remained in 2 cases after 6–12 months of anti-TB treatment. Another 14 patients did not respond to anti-TB treatment and image examinations showed an increase of PE after treatment. However, 8 of the 14 patients finally recovered after prolonged treatment but 6 patients still did not benefit from anti-TB treatment. Most of the remaining 21 patients who did not visit the hospital for reexamination in the first 1–3 months recovered from unilateral PE after treatment, however, 2 cases remained unsuccessful. Finally, 10 patients failed to benefit from empirical anti-TB treatment.
Among all the cases of confirmed diagnosis and treatment success, 72 patients received all four TB tests; Figure 3 shows the diagnostic efficacy of the four methods. As shown in the Venn diagram, only 1 case was positive in all four tests. The IGRA test was the most sensitive method for TB diagnosis with 28 cases of single positive followed by PPD test. The combination of the four tests could increase the diagnosis of TPE.

Failure analysis and diagnostic efficacy
The 10 patients who failed to benefit from this algorithm were then further evaluated; their characteristics and final diagnoses were listed in Supplementary Table S1. Among the 10 patients, 5 were misdiagnosed (1 angiosarcoma, 2 pneumonia, 1uterine adenomyosis and 1 lymphoma) and the other 5 had drug resistance. Overall, 218 cases (36 confirmed cases and 182 cases of successful treatment) were clinically diagnosed as TPE. As a result, only 5 patients (2.3%) were over-treated based on our diagnostic criteria.
To evaluate diagnostic efficacy, the ClinD algorithm was compared with other biomarkers between the two groups: the 218 final cases of TPE and 292 cases of MPE. Based on the ClinD algorithm, 11 of 36 confirmed cases were not supposed to be TPE cases and 8 of 292 MPE cases were supposed to be TPE cases. Consequently, the ROC of ClinD and other biomarkers was determined and is shown in Figure 4. Based on ROC, the AUC and the best cutoff values were determined, and the diagnostic efficacy is listed in Table 2. As shown, ClinD was the best method for distinguishing TPE from MPE with greater sensitivity and specificity than other markers followed by the level of pleural ADA. Although these markers had a satisfying sensitivity, their specificities were not acceptable.
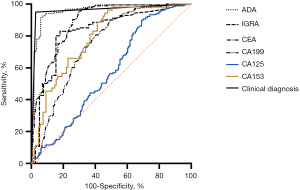
Table 2
| Markers | AUC | Cut-off | Sensitivity | Specificity | NPV | PPV | Accuracy | Youden J |
|---|---|---|---|---|---|---|---|---|
| ClinD | 0.961 | – | 0.950 | 0.973 | 0.963 | 0.963 | 0.963 | 0.922 |
| PE ADA (U/mL) | 0.959 | >26 | 0.924 | 0.941 | 0.889 | 0.961 | 0.931 | 0.866 |
| CEA (U/mL) | 0.887 | <3.2 | 0.951 | 0.697 | 0.954 | 0.684 | 0.800 | 0.648 |
| CA199 (U/mL) | 0.746 | <13.6 | 0.912 | 0.492 | 0.887 | 0.561 | 0.667 | 0.404 |
| CA125 (U/mL) | 0.568 | <255.7 | 0.911 | 0.299 | 0.832 | 0.470 | 0.547 | 0.210 |
| CA153 (U/mL) | 0.795 | <14.3 | 0.943 | 0.541 | 0.952 | 0.500 | 0.673 | 0.485 |
| IGRA (pg/mL) | 0.830 | >16.2 | 0.830 | 0.821 | 0.571 | 0.944 | 0.828 | 0.650 |
TPE, tuberculous pleural effusions; MPE, malignant pleural effusion; AUC, the area under receiver operating curve; PE, pleural effusion; ADA, adenosine deaminase; CEA, carcinoembryonic antigen; CA, carbohydrate antigen; IGRA, interferon gamma release assay.
Cause analysis
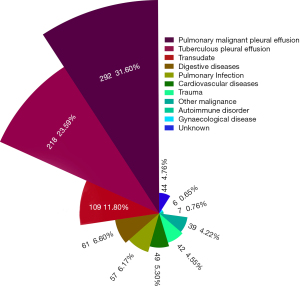
The Nightingale Rose Diagram was used to present the proportion of PE causes, shown in Figure 5. PE caused by pleural metastasis of lung cancer (31.60%) and tuberculous pleurisy (23.59%) were the two main causes of unilateral PE. Furthermore, PE in 11.80% (109 cases) of the enrolled patients was classified as transudate, which was mainly due to hypoproteinemia (e.g., liver cirrhosis, malnutrition) and heart failure, followed by digestive disease (6.60%) and pulmonary infection (6.17%). However, the causes of PE were complicated and in 4.76% of patients with unilateral PE (44 cases), the causes were unknown.
Discussion
The etiology of PE varies across different regions. In Asia, with a high burden of TB, TB infection is a major cause of PE and the rate in the present study was 26%. Although uncommon causes and distinctive types of PE are increasing, infections, malignancies, and heart failure still account for the majority of PE (15,16), which is also consistent with our results. Over the past several years, abundant evidence has increased the understanding of pleura physiology and pathophysiology as well as treatment of PE. Specifically, the increasing application of bedside thoracic ultrasound outside of radiology departments has improved patient safety during interventional pleural procedures and increased diagnostic efficacy (17). Consequently, the goal of patient care should change to a more efficient diagnosis with minimally invasive interventions that minimize the length of stay in a hospital and maximize quality of life (1).
Under current conditions, the accurate diagnosis of TPE remains a challenge for clinicians. The current Global Laboratory Initiative Advancing TB Diagnosis of WHO emphasizes the pathogenic detection of TB, recommending microscopy, culture, and molecular tests as first-line technologies. However, these methods for TPE diagnosis have several limitations. The detection rate of acid-fast staining is exceptionally low, as reported in previous studies with a sensitivity of 7–14.3% (18-20). TB-DNA detection in PE is rapid and convenient; however, the detection rate is also relatively low using either the conventional PCR test or the Xpert® MTB/RIF test. In three meta-analysis studies, respectively, conducted by Denkinger et al., Penz et al., and Shegal et al., the sensitivity ranged from 37% to 51.4% (21-23). Similarly, the total number of cases positive for conventional PCR, culture and smear in the present study was only 36 of 218 cases (16.5%). Furthermore, nucleic acid contamination can cause some false positives and detecting TB-DNA in PE takes longer than tests performed on other sample types, therefore this test is not recommended. For immunological tests, IGRA test was suggested for active TB diagnosis. In several meta-analyses, the diagnostic performance of IGRAs using T-SPOT.TB® (Oxford Imunotec, Oxfordshire, UK) or QuantiFERON® (Cellestis, Carnegie, Australia) showed the sensitivity and specificity of IGRAs were 77% and 78%, respectively (24,25); the sensitivity and specificity were 83% and 82%, respectively, in the present study. ADA in PE is considered a useful biomarker for TPE, with a sensitivity of 88–98% in many studies (26,27). However, high levels of ADA can also be observed in pleural fluid from patients with parapneumonic effusions, empyema, malignancy, or rheumatoid disease (28), and the best ADA cut-off point for TPE remains controversial (29). In recent years, several new markers have been developed, such as TNF-α (30), interleukin-33 (8), and cell-free DNA (31). However, these markers are far from being widely used in clinics. In conclusion, none of these tests alone can achieve a clear and satisfactory diagnosis of TPE.
A comprehensive analysis combining different detecting methods is then suggested. Some studies have been performed but mostly focused on the combination of two markers. Keng et al. concluded that the combination of ADA and IFN-γ had a specificity of 100% with high sensitivity (32). Tang et al. examining IGRA and widely-used biomarkers, found that pleural IGRA together with ADA and CEA provided the best efficacy for differentiating TPE from MPE (33). However, the importance of pleural biopsy, especially for patients with negative microbiological and immunological results, was emphasized in other studies (10,11). In a present study, the combination of microbiological and immunological tests was shown necessary. As shown in the Venn diagram, more than one immunological test should be recommended. The sensitivity of the combination was 95% and the specificity 97.3%, respectively. The DeLong test showed that AUC of the combination was significantly higher than ADA alone, indicating the efficiency of the combination was satisfactory.
Globally, the prevalence of TB was, in general, negatively associated with income levels. Underdevelopment limits the wide application of sophisticated testing methods. In primary hospitals, some invasive methods can rarely be used and under these circumstances, diagnosis is made insufficiently. In many suspected cases of negative microbiological results, the starting point of treatment is confusing. Consequently, the establishment of this diagnostic algorithm may help in the early diagnosis of TB and TPE. In this protocol, except for confirmed diagnoses, the start of treatment was also recommended for clinically diagnosed patients. Treatment could also be started for suspected cases after a comprehensive analysis. Therefore, this diagnostic algorithm might be a potentially effective complement in regions of high TB burden based on our experience and statistical results.
Additionally, the determination of a clear diagnosis should be emphasized. In the present study, the final confirmation was based on a good response to anti-TB chemotherapy during 1 year of follow-up (34), thus, M. tuberculosis or its DNA was not detected. Inevitably, some cases were over-diagnosed and overtreated. Furthermore, drug resistance is a major consideration in TB, however, this diagnostic algorithm does not include this aspect. The appropriate time for drug resistance testing should be further studied. Besides, as a retrospective study, incomplete data and selection bias were still the big limitations. Further large-scale, multicenter, and prospective studies should be carefully designed to confirm this algorithm.
In conclusion, this clinical diagnostic algorithm for TB was an efficient and available method for TPE diagnosis, could help with the early diagnosis and treatment of suspected patients with TPE, and should be implemented in regions of high prevalence of TB. This algorithm should be further developed with a focus on the appropriate time for drug resistance testing.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (81800088).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1532/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1532/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1532/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1532/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of the General Hospital of Western Theater Command. As a retrospective study, informed consent was not sought from all enrolled patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Perricone G, Airoldi A, Vangeli M. Pleural Disease. N Engl J Med 2018;378:1753-4. [Crossref] [PubMed]
- Light RW, Lee YCG. Textbook of Pleural Diseases, Third Edition. Pulmonary Medicine 2014.
- Ferreiro L, Toubes ME, San José ME, et al. Advances in pleural effusion diagnostics. Expert Rev Respir Med 2020;14:51-66. [Crossref] [PubMed]
- Haas AR, Sterman DH. Advances in pleural disease management including updated procedural coding. Chest 2014;146:508-13. [Crossref] [PubMed]
- Porcel JM, Esquerda A, Vives M, et al. Etiology of pleural effusions: analysis of more than 3,000 consecutive thoracenteses. Arch Bronconeumol 2014;50:161-5. [Crossref] [PubMed]
- Pang Y, An J, Shu W, et al. Epidemiology of Extrapulmonary Tuberculosis among Inpatients, China, 2008-2017. Emerg Infect Dis 2019;25:457-64. [Crossref] [PubMed]
- Light RW. Update on tuberculous pleural effusion. Respirology 2010;15:451-8. [Crossref] [PubMed]
- Al-Aarag AH, Kamel MH, Abdelgawad ER, et al. Diagnostic role of interleukin -33 in the differentiation of pleural effusions especially tuberculous and malignant effusions. BMC Pulm Med 2019;19:114. [Crossref] [PubMed]
- Antonangelo L, Faria CS, Sales RK. Tuberculous pleural effusion: diagnosis & management. Expert Rev Respir Med 2019;13:747-59. [Crossref] [PubMed]
- Wong CF. Early diagnosis of tuberculous pleural effusion: apart from pleural fluid adenosine deaminase, pleural biopsy still has a role. Hong Kong Med J 2018;24:316-7. [Crossref] [PubMed]
- Huo Z, Yang M, Chen J, et al. Improved early diagnosis of difficult cases of tuberculous pleural effusion by combination of thoracoscopy with immunological tests. Int J Infect Dis 2019;81:38-42. [Crossref] [PubMed]
- Porcel JM. Biomarkers in the diagnosis of pleural diseases: a 2018 update. Ther Adv Respir Dis 2018;12:1753466618808660. [Crossref] [PubMed]
- Shi XC, Tan YT, Zhang LF, et al. Evaluation of laboratory diagnostic approaches for tuberculous pleurisy. Infect Dis (Lond) 2019;51:384-6. [Crossref] [PubMed]
- China NHaFPCotPsRo. Diagnosis for pulmonary tuberculosis. 2017. Available online: http://wsbz.nhc.gov.cn/wsbzw/article/StandardLibrary/4848e49b20644f9c01206451a5d6000b/2019/2/01033.html (Accessed 06-08 2020).
- Walker S, Maskell N. Identification and management of pleural effusions of multiple aetiologies. Curr Opin Pulm Med 2017;23:339-45. [Crossref] [PubMed]
- Ryu JH, Tomassetti S, Maldonado F. Update on uncommon pleural effusions. Respirology 2011;16:238-43. [Crossref] [PubMed]
- Corcoran JP, Hallifax R, Rahman NM. Advances in the management of pleural disease. Expert Rev Respir Med 2013;7:499-513. [Crossref] [PubMed]
- Choi H, Chon HR, Kim K, et al. Clinical and Laboratory Differences between Lymphocyte- and Neutrophil-Predominant Pleural Tuberculosis. PLoS One 2016;11:e0165428. [Crossref] [PubMed]
- Han M, Xiao H, Yan L. Diagnostic performance of nucleic acid tests in tuberculous pleurisy. BMC Infect Dis 2020;20:242. [Crossref] [PubMed]
- Bielsa S, Acosta C, Pardina M, et al. Tuberculous Pleural Effusion: Clinical Characteristics of 320 Patients. Arch Bronconeumol 2019;55:17-22. (Engl Ed). [Crossref] [PubMed]
- Penz E, Boffa J, Roberts DJ, et al. Diagnostic accuracy of the Xpert(R) MTB/RIF assay for extra-pulmonary tuberculosis: a meta-analysis. Int J Tuberc Lung Dis 2015;19:278-84. i-iii. [Crossref] [PubMed]
- Sehgal IS, Dhooria S, Aggarwal AN, et al. Diagnostic Performance of Xpert MTB/RIF in Tuberculous Pleural Effusion: Systematic Review and Meta-analysis. J Clin Microbiol 2016;54:1133-6. [Crossref] [PubMed]
- Seo YS, Kang JM, Kim DS, et al. Xpert MTB/RIF assay for diagnosis of extrapulmonary tuberculosis in children: a systematic review and meta-analysis. BMC Infect Dis 2020;20:14. [Crossref] [PubMed]
- Pang CS, Shen YC, Tian PW, et al. Accuracy of the interferon-gamma release assay for the diagnosis of tuberculous pleurisy: an updated meta-analysis. PeerJ 2015;3:e951. [Crossref] [PubMed]
- Aggarwal AN, Agarwal R, Gupta D, et al. Interferon Gamma Release Assays for Diagnosis of Pleural Tuberculosis: a Systematic Review and Meta-Analysis. J Clin Microbiol 2015;53:2451-9. [Crossref] [PubMed]
- Aggarwal AN, Agarwal R, Sehgal IS, et al. Adenosine deaminase for diagnosis of tuberculous pleural effusion: A systematic review and meta-analysis. PLoS One 2019;14:e0213728. [Crossref] [PubMed]
- Gui X, Xiao H. Diagnosis of tuberculosis pleurisy with adenosine deaminase (ADA): a systematic review and meta-analysis. Int J Clin Exp Med 2014;7:3126-35. [PubMed]
- Skouras VS, Kalomenidis I. Pleural fluid tests to diagnose tuberculous pleuritis. Curr Opin Pulm Med 2016;22:367-77. [Crossref] [PubMed]
- Servonnet A, Frederic C, Fargeau F, et al. Pleural adenosine deaminase cut-off value for the diagnosis of tuberculous pleural effusion using the Diazyme assay. Hong Kong Med J 2018;24:207. [Crossref] [PubMed]
- Damayanti N, Yudhawati R. The comparison of pleural fluid TNF-alpha levels in tuberculous and nontuberculous pleural effusion. Indian J Tuberc 2020;67:98-104. [Crossref] [PubMed]
- Yang X, Che N, Duan H, et al. Cell-free Mycobacterium tuberculosis DNA test in pleural effusion for tuberculous pleurisy: a diagnostic accuracy study. Clin Microbiol Infect 2020;26:1089.e1-6. [Crossref] [PubMed]
- Keng LT, Shu CC, Chen JY, et al. Evaluating pleural ADA, ADA2, IFN-gamma and IGRA for diagnosing tuberculous pleurisy. J Infect 2013;67:294-302. [Crossref] [PubMed]
- Tang Y, Zhang J, Huang H, et al. Pleural IFN-gamma release assay combined with biomarkers distinguished effectively tuberculosis from malignant pleural effusion. BMC Infect Dis 2019;19:55. [Crossref] [PubMed]
- Villena Garrido V, Cases Viedma E, Fernández Villar A, et al. Recommendations of diagnosis and treatment of pleural effusion. Update. Arch Bronconeumol 2014;50:235-49. [Crossref] [PubMed]




