Indocyanine green fluorescence-navigated thoracoscopy versus traditional inflation-deflation approach in precise uniportal segmentectomy: a short-term outcome comparative study
Introduction
With the broad application of low-dose spiral computed tomography (CT), more and more lung cancer has been detected as small lung nodules, especially ground-glass nodules (GGN). Segmentectomy could achieve complete tumor resection while preserving as much functioning pulmonary tissue as possible. However, compared with lobectomy, precise segmentectomy faced more challenges. On the one hand, anatomical variations are common in the bronchi and blood vessels of the lung segments. On the other hand, the lung segments are separated by the intersegmental boundary line that varies from person to person. Although there are multiple ways to identify intersegmental boundary lines during segmentectomy, two main methods have been widely used to create an intersegmental boundary: inflation-deflation and near-infrared fluorescence imaging with intravenous indocyanine green (ICG) (1).
Due to the highly restricted space of the uniportal video-assisted thoracoscopic surgery (VATS) operation, the ICGF-based method has been considered to be superior to the traditional inflation-deflation method in identifying the intersegmental boundary line. ICGF-based navigation can provide a clear surgical field by allowing maintenance of the deflated state of the lung and can achieve a high rate of accurate identification of the intersegmental line. Another important issue with segmentectomy is how to achieve an accurate resection. Modified uniportal VATS segmentectomy has been performed to solve these issues. For instance, Mimics software has been successfully used to perform a three-dimensional (3D) reconstruction of the lung before surgery, which facilitates the accurate identification of the bronchi and blood vessels of the lung segment where the lung nodule is located. As such, combined ICGF-based method and Mimics software-based 3D reconstruction is expected to enable the accurate and quick identification of the intersegmental plane. Here, we describe our experience in applying ICGF-based method and Mimics software-based 3D reconstruction for uniportal VATS segmentectomy and its advantage (2,3). We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-292/rc).
Methods
Study design and patients
Study design
The clinical and imaging data of 200 patients undergoing uniportal VATS segmentectomy under ICGF-navigated (N=100) or inflation-deflation (N=100) methods were analyzed, retrospectively, at Shanghai Chest Hospital from December 2018 to August 2020.
Patients
This study was approved by the Institutional Review Board of Shanghai Chest Hospital (#KS1972). All patients had signed informed consent to include their clinical data and specimens in our Lung Biobank and for use in research projects, according to the recommendation of the Institutional Review Board of Shanghai Chest Hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
The inclusion criteria
(I) aged 18–75 years; (II) with adenocarcinoma in situ sized <2 cm, with a GGO proportion of 50% or more or tumor doubling time >400 days; (III) patients had not received any preoperative neoadjuvant treatment such as chemotherapy, radiotherapy, or traditional Chinese medicine therapies; (IV) a preoperative assessment according to the National Comprehensive Cancer Network (NCCN) guidelines confirmed that the patient’s cardiopulmonary function could tolerate the surgery.
Exclusion criteria
There were no distant metastases, serious underlying diseases, or any other contraindications to surgery. Both inclusion and exclusion criteria were the same in the two groups. The general information was summarized in Table 1.
Table 1
| Variables | Data |
|---|---|
| General information | |
| Age (years) (mean ± SD) | 54±11.25 |
| Gender (male/female) (n) | 53/47 |
| Tumor size (mm) (mean ± SD) | 12.9±3.04 |
| Surgical sites (n) | |
| Left upper lobe apicoposterior segment | 15 |
| Left upper lobe anterior segment | 10 |
| Left upper lobe inherent segment | 4 |
| Left upper lobe lingual segment | 7 |
| Left lower lobe dorsal segment | 9 |
| Right upper lobe apical segment | 11 |
| Right upper lobe posterior segment | 12 |
| Right upper lobe anterior segment | 13 |
| Right lower lobe dorsal segment | 8 |
| Right lower lobe basal segment | 11 |
SD, standard deviation.
Interventions
A thin spiral CT (slice thickness: 1 mm) scan was performed preoperatively. Based on the CT images, the branches of the trachea, pulmonary artery, and pulmonary vein in the target segment where the lung nodule was located were reconstructed in three dimensions using Mimics software (Materialise, Beijing HuanZhongRuiChi Technology Co., Ltd., Beijing, China). All patients were found to have operable peripheral lung GGNs on health check-ups. Segmentectomy was feasible for these patients.
Operations of ICGF-based method group
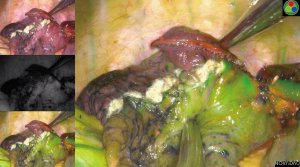
The patient was placed on the unaffected side under general double-lumen endotracheal intubated anesthesia. A 4.0-cm incision was created in the 5th intercostal space between the anterior axillary line and the midaxillary line at the diseased side. The incision protector was placed at the observation and operation port. During the surgery, one-lung ventilation was applied to the unaffected side. The uniportal VATS segmentectomy was performed as scheduled. First, the pulmonary artery, pulmonary vein, and bronchus in the target segment were fully mobilized sequentially according to the reconstructed 3D images and transected separately with a cutter/stapler; alternately, these structures were clamped with a Hem-o-lok clip, followed by ultrasonic knife dissection. The intersegmental veins were preserved. Then, the anesthesiologist injected 5 mL of ICG into the peripheral vein. The fluorescence thoracoscope mode was switched from “Normal mode” to “Fluorescence mode” to observe the color border of the lung surface. The intersegmental plane was judged according to the color border. After the intersegmental plane was marked with argon plasma coagulation or an electrocoagulation hook, the target segment was completely resected using a cutter/stapler along the plane. The lymph nodes were sampled, and the target lung segments were sent for frozen section examination. After complete hemostasis, a 24-gauge closed chest tube and a drainage tube were left in place.
Operations of inflation-deflation method group
In addition, 100 patients who underwent uniportal VATS segmentectomy with the inflation-deflation method in our center during the same period were retrospectively selected as the control group. The same inclusion criteria, surgical indications, number of selected target lung segments, preoperative reconstruction method, anesthesia method, and surgical process were applied in this group. However, the intersegmental plane was judged by using the inflation-deflation method. After the pulmonary artery, pulmonary vein, and bronchus in the target lung segment were transected, the anesthesiologist was asked to inflate the lung with pure oxygen. When the lung was completely re-expanded, one-lung ventilation on the healthy side was continued. Twenty minutes later, the junction line between the target segment, which had not collapsed, and the non-target segment, which had completely collapsed, was identified as the intersegmental plane.
Assessments
After surgery, the postoperative drainage volume, postoperative air leakage time, postoperative drainage tube retention time, length of hospital stay, bronchopleural fistula, pulmonary infection/pleural effusion, and tumor recurrence/metastasis were recorded. The operative time was compared between the ICGF and the control groups, and intraoperative blood loss was recorded.
Statistical analysis
Normally distributed continuous variables were presented as the mean ± standard deviation (SD) or median and range. Categorical variables were shown as numbers and percentages. The operative time between the ICGF and control groups was compared using the two-sided Student’s t-test for continuous variables. The data summary and statistical analysis were performed using SPSS software (version 19.0; IBM-SPSS Inc., Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Results
In the ICGF group, after the pulmonary artery, pulmonary vein, and bronchus in the target segment were mobilized and transected, ICG injection via a peripheral vein immediately revealed that all lung tissues except the target segment turned green, while the target segment remained in its original color; thus, a clear boundary was visible. The intersegmental plane was marked with argon plasma coagulation or an electrocoagulation hook, and the target segment was completely removed along this plane with a cutter/stapler (Figures 1,2). Lymph node sampling was performed, and the intraoperative frozen section pathology confirmed the presence of adenocarcinoma in situ. Standard segmentectomy was completed in all cases. No major bleeding was noted. The intraoperative vital signs were normal, including stable heart rate, rhythm, and blood pressure. The surgery was smooth. No postoperative complications occurred, such as hemoptysis, pulmonary artery embolism, pulmonary atelectasis, bronchopleural fistula, hypoxemia, or celiac disease. Postoperative pathology revealed negative mediastinal and intrapulmonary lymph nodes, and all patients were discharged successfully. Postoperative chest CT scans at 1 month, 3 months, 6 months, and 1 year showed good expansion of the remnant lungs and normal lung function. No recurrence or metastasis of the tumor was observed (Tables 2,3).
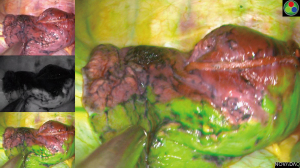
Table 2
| Group | Operative time (minutes) (mean ± SD) | Patient number | P value |
|---|---|---|---|
| ICGF group | 90±11.46 | 100 | <0.001 |
| Inflation-deflation group | 118±10.59 | 100 |
P value was calculated using a two-sided Student’s t-test. ICGF, indocyanine green fluorescence; SD, standard deviation.
Table 3
| Intra- and post-operative indicators | Data |
|---|---|
| Operative time (minutes) (mean ± SD) | 90±11.46 |
| Intraoperative blood loss (mL) (mean ± SD) | 94±19.59 |
| Postoperative drainage volume (mL) (mean ± SD) | 215±56.64 |
| Duration of postoperative air leak (days) (mean ± SD) | 1.9±0.45 |
| Duration of postoperative drainage tube retention (days) (mean ± SD) | 2.45±0.50 |
| Duration of hospital stay (days) (mean ± SD) | 3.85±0.73 |
| Bronchopleural fistula (n) | 0 |
| Peritoneal effusion (%) | 1 (0.01) |
| Pulmonary infection (%) | 2 (0.02) |
SD, standard deviation.
Discussion
GGNs are manifestations of early-stage lung cancer, with relatively low harm and a high probability of surgical cure. An increasing number of retrospective studies support the feasibility of performing a less extensive sublobar resection in these patients, preserving as much normal lung tissue as possible while completely removing the tumor; thus protecting lung function, reducing trauma, and improving quality of life (4-7). Sublobar resection includes two procedures: wedge resection and segmentectomy. Although wedge resection is easy to perform, it has limited indications and is only applicable to resection of pulmonary nodules located in the peripheral one-third of the lung; furthermore, it cannot remove the intrapulmonary lymph nodes. Thus, wedge resection is feasible only for a small proportion of patients with strict indications. Pulmonary segmentectomy has unique advantages. On the one hand, it has an anatomical basis, in which the bronchi and pulmonary vessels in each lobe are subdivided into segmental bronchi and segmental vessels, and there are intersegmental planes separating the different lung segments; on the other hand, segmentectomy enables not only the removal of pulmonary tumors but also the dissection of intrapulmonary lymph nodes (8-10).
Therefore, in recent years, pulmonary segmentectomy has become a hot research topic in thoracic surgery. However, compared with lobectomy, precise segmentectomy is far more challenging. First, segmentectomy requires the dissection of the lung parenchyma, and the surgical trauma surface is prone to bleeding, making the operation more difficult. Second, anatomical variations are common in the bronchi and blood vessels of the lung segments. If the operation is entirely based on the operator's experience, the bronchi and blood vessels may be transected by mistake, which occurs frequently and unpredictably; as a result, patients may experience serious complications such as bleeding, air leak, hemoptysis, and even death. In addition, the intersegmental plane that separates the lung segments varies from person to person, and the presence of numerous communicating branches of lung tissue among lung segments results in no absolute intersegmental plane, which also poses an obstacle to precise segmental resection (10,11).
Segmentectomy based on a surgeon’s personal experience is difficult to standardize, and the learning curve is long. Postoperative complications, such as hemoptysis and air leak, frequently occur, and the long-term outcome of residual lung re-expansion is not ideal. Thus, achieving precise lung segment resection for early-stage lung cancer poses a significant challenge to surgeons. Preoperatively, reconstruction can be performed using Mimics software, which accurately locates the lung segment where the lung nodule is located and enables 3D reconstruction of the trachea and blood vessels in the target lung segment. This allows the trachea and blood vessels to be correctly identified intraoperatively, thus ensuring the precise mobilization and transection of these structures. After transection of the trachea and blood vessels, intersegmental plane determination is challenging in precise segmentectomy. Ideally, a precise resection can remove all lung tissue in the target segment and preserve all tissue outside the target segment. However, there are no clear demarcation lines among lung segments due to a large number of airway passages among the lung segments. The inflation-deflation method and modified inflation-deflation method are two conventional strategies, which often require a lengthy waiting time (typically 20–30 minutes). In our retrospective analysis, we also found that the operative time to complete lung segment resection was significantly longer in the control group (using the inflation-deflation method) than in the ICGF group, mainly because of the need to wait more than 20 minutes to collapse the lung after expansion in the control group. In contrast, the intersegmental plane could be determined within seconds by applying fluorescence thoracoscopy in the ICGF group. In particular, the result of intersegmental plane determination by the inflation-deflation method is unsatisfactory for patients with emphysema, which makes precise segmentectomy extremely difficult (11). In addition, both the inflation-deflation method and the modified inflation-deflation method led to the incomplete collapse of some lung tissues, affecting the surgical field and prolonging the operative time.
In recent years, the fluorescence imaging technique has been used in liver segment resection by intravenous injection of ICG, a specific visualizing agent that is a sterile, water-soluble tricarbocyanine dye. ICG rapidly binds to plasma proteins after intravenous injection, with a peak spectral absorption at 800–810 nm in blood plasma or blood. The distribution area of ICG can be seen through a special fluorescence endoscope for real-time visualization (12). During segmentectomy, ICG is injected through a peripheral vein after accurate transection of the pulmonary artery in the target segment; 20–30 seconds later, the fluorescence endoscope shows lung tissue outside the target segment has turned a clear green fluorescent color while the target segment has no fluorescent distribution, allowing rapid determination of the intersegmental plane (13,14). In our current retrospective analysis, we found that fluorescence-navigated uniportal VATS segmentectomy not only shortened the operative time but also had no postoperative complications. No tumor recurrence or metastasis was seen in multiple postoperative follow-up visits. Therefore, the intersegmental planes were accurately determined, and the resection of the target lung segments could ensure sufficient surgical margins.
The experience of ICGF-navigated uniportal VATS segmentectomy in our center may be summarized as follows: (I) the indications for lung segmentectomy must be strictly followed according to the guidelines; (II) 3D reconstruction must be performed preoperatively to locate the lung segment in which the nodule is located and to reconstruct the trachea and related blood vessels in the target segment to observe whether there are anatomical variations of the trachea and blood vessels; (III) the trachea and related vessels of the target segment must first be mobilized and transected. Any mistransection, undertransection, or overtransection, especially for the pulmonary artery, must be avoided as these errors can result in the wrong confirmation of the intersegmental plane of the target segment after the ICG is injected via the peripheral vein; (IV) ICG may be intravenously injected at a dose of 4–5 mL, although the color development is poor with lower doses. The color change occurs 20–30 seconds after injection and can last 3–5 minutes; (V) intravenous injection of ICG should not be too fast; otherwise, the intersegmental plane may not be detected (15); and (VI) before the intravenous injection of ICG, the fluorescence thoracoscopy is switched from normal mode to fluorescence mode; after the injection, lung tissue other than the target segment turns green within a short period. The intersegmental plane should be immediately marked with argon plasma coagulation or an electrocoagulation hook—otherwise, the lung tissue in the target segment may also turn green in a few minutes, which may be due to the diffusion of the ICG along the communicating branches.
Preoperative 3D reconstruction combined with uniportal VATS for standard lung segmentectomy has the following advantages: (I) it maximizes the preservation of lung function while removing lung lesions; (II) it mobilizes the trachea and blood vessels in strict accordance with the anatomical structure of the lung segment and resects the target lung segment along the actual intersegmental plane, which reduces the occurrence of postoperative complications; (III) it shortens the operative time because it only takes 20–30 seconds to determine the intersegmental plane using the fluorescence thoracoscopy (in contrast, the inflation-deflation method requires at least 15–20 minutes to determine the intersegmental plane); (IV) the inflation-deflation method affects the surgical field after re-expansion, but the fluorescence thoracoscopy does not. In addition, the inflation-deflation method cannot be applied in surgeries for patients with co-existing emphysema, whereas fluorescence thoracoscopy typically will not be affected by emphysema; (V) after the intersegmental plane is accurately determined, standard lung segmentectomy can be performed along the intersegmental plane, which reduces postoperative complications, such as air leak, hemoptysis, and pulmonary atelectasis. Furthermore, it avoids the unnecessary loss of lung function due to excessive resection of lung tissue; and (VI) ICG has no known metabolites in the body and is excreted in its original form, which is not harmful to humans (16,17).
Conclusions
Uniportal fluorescence thoracoscopy combined with preoperative 3D reconstruction is valuable for precise lung segmentectomy. It has excellent results with a short operative time, simple, safe, reliable surgery, and few postoperative complications. Thus, this method can be widely applied in lung segment surgeries.
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This research was supported by the Multidisciplinary Collaborative Innovation Project of Shanghai Chest Hospital and the Science and Technology Development Project of Shanghai Municipality (No.19411963900 to Feng Yao), and the Medical-Engineering Interdisciplinary Research Project of Shanghai Jiao Tong University (No. YG2021QN128 to Zhexin Wang).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-292/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-292/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-292/coif). FY reports that this research was funded by the Multidisciplinary Collaborative Innovation Project of Shanghai Chest Hospital and the Science and Technology Development Project of Shanghai Municipality (No. 19411963900). ZW reports that this research was funded by the Medical-Engineering Interdisciplinary Research Project of Shanghai Jiao Tong University (No. YG2021QN128). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Institutional Review Board of Shanghai Chest Hospital (No. #KS1972). Informed consent was obtained from all participants involved in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang J, Xu X, Wen W, et al. Modified method for distinguishing the intersegmental border for lung segmentectomy. Thorac Cancer 2018;9:330-3. [Crossref] [PubMed]
- Roman M, Labbouz S, Valtzoglou V, et al. Lobectomy vs. segmentectomy. A propensity score matched comparison of outcomes. Eur J Surg Oncol 2019;45:845-50. [Crossref] [PubMed]
- Fu HH, Feng Z, Li M, et al. The arterial-ligation-alone method for identifying the intersegmental plane during thoracoscopic anatomic segmentectomy. J Thorac Dis 2020;12:2343-51. [Crossref] [PubMed]
- Duan J, Cai H, Huang W, et al. Bronchial Sleeve Resection with Complete Pulmonary Preservation: A Single-Center Experience. Cancer Manag Res 2020;12:12975-82. [Crossref] [PubMed]
- Ghaly G, Kamel M, Nasar A, et al. Video-Assisted Thoracoscopic Surgery Is a Safe and Effective Alternative to Thoracotomy for Anatomical Segmentectomy in Patients With Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:465-72; discussion 472. [Crossref] [PubMed]
- Lex JR, Naidu B. In patients with resectable non-small-cell lung cancer, is video-assisted thoracoscopic segmentectomy an appropriate alternative to video-assisted thoracoscopic lobectomy? Interact Cardiovasc Thorac Surg 2016;23:826-31. [Crossref] [PubMed]
- Wang X, Guo H, Hu Q, et al. Pulmonary function after segmentectomy versus lobectomy in patients with early-stage non-small-cell lung cancer: a meta-analysis. J Int Med Res 2021;49:3000605211044204. [PubMed]
- Wang P, Wang S, Liu Z, et al. Segmentectomy and Wedge Resection for Elderly Patients with Stage I Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. J Clin Med 2022;11:294. [Crossref] [PubMed]
- Rao S, Ye L, Min L, et al. Two-Way: A Novel Method for Identifying the Anatomy During Uniportal Thoracoscopic Segmentectomy. Ann Thorac Surg 2020;110:e441-3. [Crossref] [PubMed]
- Nakazawa S, Shimizu K, Mogi A, et al. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg 2018;66:81-90. [Crossref] [PubMed]
- Majlesara A, Golriz M, Hafezi M, et al. Indocyanine green fluorescence imaging in hepatobiliary surgery. Photodiagnosis Photodyn Ther 2017;17:208-15. [Crossref] [PubMed]
- Nakaseko Y, Ishizawa T, Saiura A. Fluorescence-guided surgery for liver tumors. J Surg Oncol 2018;118:324-31. [Crossref] [PubMed]
- Chiu CH, Chao YK, Liu YH, et al. Clinical use of near-infrared fluorescence imaging with indocyanine green in thoracic surgery: a literature review. J Thorac Dis 2016;8:S744-8. [Crossref] [PubMed]
- Newton AD, Predina JD, Nie S, et al. Intraoperative fluorescence imaging in thoracic surgery. J Surg Oncol 2018;118:344-55. [Crossref] [PubMed]
- Misaki N, Tatakawa K, Chang SS, et al. Constant-rate intravenous infusion of indocyanine green leading to high fluorescence intensity in infrared thoracoscopic segmentectomy. JTCVS Tech 2020;3:319-24. [Crossref] [PubMed]
- Fan L, Yang H, Yu L, et al. Multicenter, prospective, observational study of a novel technique for preoperative pulmonary nodule localization. J Thorac Cardiovasc Surg 2020;160:532-539.e2. [Crossref] [PubMed]
- Mun M, Okumura S, Nakao M, et al. Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy. J Vis Surg 2017;3:80. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)